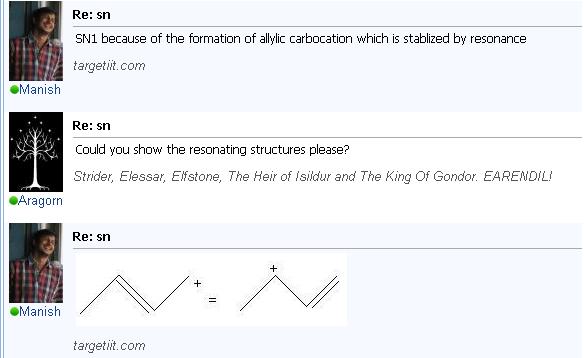
Because of the stability of the allylic carbocation.
HOPE I'M CORRECT [7]
why allylic halide is more reactive than alkyl halide in williamson's synthesis of ether ?????
-
UP 0 DOWN 0 0 10

10 Answers
Anirudh Narayanan
·2009-01-13 03:04:48
greatvishal swami
·2009-01-13 03:05:58
dekho gi williamson ether follows SN2 mechanism now in allylic ether branching is less due to doublr bond also the double bond makes it more compact & polar
greatvishal swami
·2009-01-13 03:07:00
stability ka koi chakkar nahi hai ani
it strictly follows SN2 mechanism
Anirudh Narayanan
·2009-01-14 01:19:15
See, even stability of carbocation is a reason for reactivity of allylic halide. But you said that was not so. That's why I posted the above reply [1]