 29
29Nice idea Anand.. : )
Dipole Moments
CH3Cl > CHCl3
CH3F < CH3Cl ...
CCl3CHO can exist in it's hydrated form ie CCl3CH(OH)2
Cyclopropanone also exists in it's hydrate form due to large angle
Formaldehyde can exist in it's hydrate form..
Though F is more electronegative than Cl but still deactivating power of Cl > F
CF3 substitued on benzene will act as a meta directing grp..
see this thread one more exception given here :
http://www.targetiit.com/iit-jee-forum/posts/will-it-undergo-iodoform-reaction-15334.html
 13
13Ok, so SUMMARISING ALL ABSURD THINGS GIVING/NOT GIVING TOLLEN's
Those which give-
*Benzaldehyde
*Formic Acid (gives Fehling as well)
*Fructose
*Alpha-Hydroxy Ketones
*Terminal Alkynes (Not a silver mirror but a white/yellow ppt.)
*Glyoxillic acid (frm ankur's post abv,,,though i dunno wht it is :P)
Those which don't-
*Tertiary Alcohols
*Compounds with any lone pair giving atom adjacent to the Carbonal Group (like amides, acid halides, esters etc..) (THis expl. is more fr Iodofrom)
(Also, see #4 abv,fr Haloform reac.n exceptions)
Tell me if there are more to add...we'll add here itself ; sab ek-saath [1]
 39
39LiAlH4 se yaad aaya...it can reduce all groups except double/triple bonds. HOWEVER, if a phenyl group is present beta to the double bond, it reduces the double bond as well.
 1
1Not only, LiAlH4, NaBH4 works similarly.. but I guess, NaBH4 reduces double bond when double bond is in conjugation.
and...
Either Sodium in liquid NH3 or LiAlH4 are considered best reagents to convert alkyne to alkene.. coz they don't reduce them further.
 29
29NR3+ is more deactivating than NH3+...
2,4 DNP is also known as Brady's reagent
Ester Hydrolysis in the presence of acid is a pseudo first order reaction and in the presence of base is a second order reaction..
3 Hydroxy Butan-2one can show positive Tollen' s and Fehling's solution test..
C≡O is more stable than C≡C ..
 106
106to last post
not only 3-hydroxy butan-2-one.. but all α-hydroxy ketones give tollen's and fehling's test.
 1
1p-hydroxy benzoic acid less acidic to C6H5COOH but o-hydroxy salicylic acid more than benzoic acid.
Presence of +M directing group decreases acidic character due to increasing -ve charge on carboxylate ion. In salicylic acid exception is due to Intara molecular H-bonding,which stbilizes o-hydroxy benzoate ion.
 1
1add to last post
also explained by ortho effect......a effect in which an ortho group increase acidity and decreases basicity
 29
29Terminal Alkynes give Tollen's test
Twist boat format of cyclohexane is chiral
NH2C2H5 and NH(CH3)2 are functional isomers i thot they will be metamers but acc. to Bansal assignments they are functional isomers
 1
1glyoxilic acid gives tollens' test but not fehlings.
reaction of NH3 and formaldehyde gives urotropine.
 1
1glycol reacts with dehydrating agents differently,
with (ZnCl2 + heat), glycol dehydrates to form acetaldehyde
with (H3PO4 + heat), glycol dehydrates to form diethylene glycol.
 1
1H2CrO4 DOES NOT OXIDISE DOUBLE AND TRIPLE BONDS LIKE KMnO4 OR K2CR2O7
 1
1CH≡CH ON OZONOLYSIS HYDROLYSIS GIVES GLYOXAL AND FORMIC ACID
 1
1Also a good exception.
Both twist boat and half chair form of cyclohexane are chiral
 1
1And also schiff base give tollen test+ve.
Schiff bases= - C=N-OH
 1
1Also methanol is exceptionally more acidic than water. And also methoxide is better leaving group than hydroxide.
 1
1please keep posting guys. its very helpfull for us. the exams are near. also please make a similar one for inorganic. thanks in advance ;p
Anonymous
·2017-10-22 19:53:10
LiAlH4 gives azobenzene (Ph-N=N-Ph) on reaction with aniline(Ph-NH2).
 39
39An exception to pinacol-pinacolone rearrangement -:
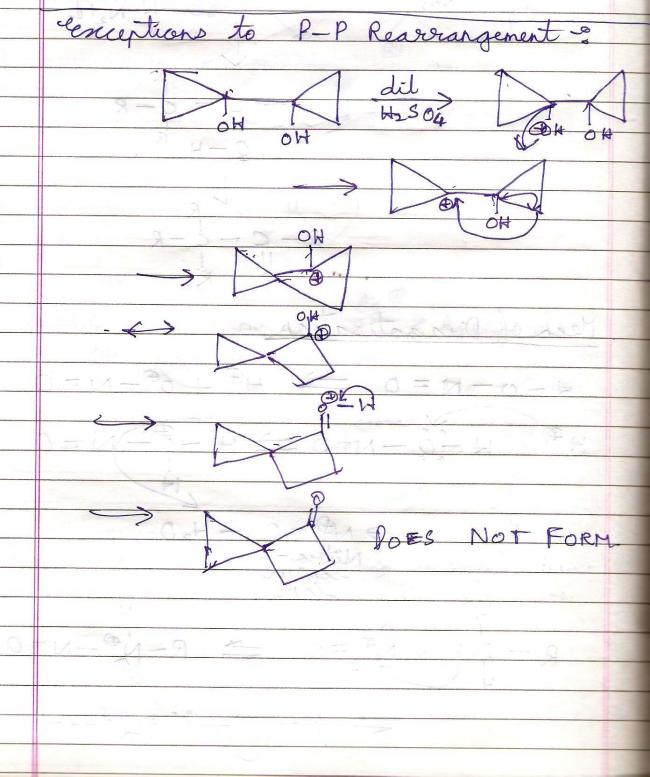
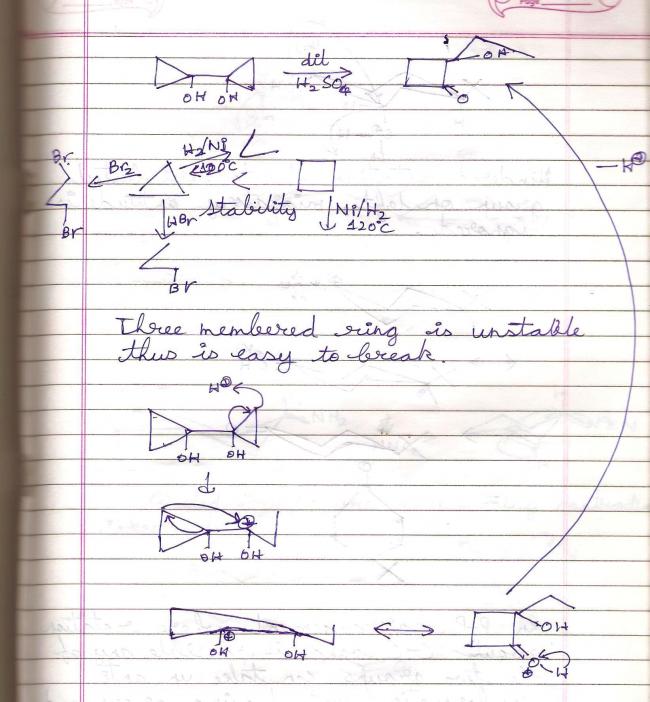
 39
39Weird Compounds which give/don't give iodoform -:

 29
29Pyridine behaves like a deactivated benzene and does not undergo electrophilic substituions easily...there are more chences of nucleophilic substitution in case of pyridine..
whereas pyrolle behave like an activated benzene and undergo electrophilic reactions faster than benzene and the attack is generally on the carbon just next to Nitrogen atom...
Decreasing order of acidic character
Ph-SeH > Ph-SH > Ph-OH
thiophenol Phenol
p-NitroPhenol > o-NitroPhenol
o-FluoroPhenol > p-FluoroPhenol
Aniline does not undergo Friedal Crafts reactions..
Aromatic character of thiophene > furan
and pyrole > furan
 1
1Nice Thread! Keep it going.
I'll not talk of exceptions here coz I can't remember one, but these are from my notes.
H2/Metal reduces every carbonyl, carboxylic group but H2/Pd neither reduces COOH group nor -CO- group.
In glucose, cystosine and thymine are pyrimidine bases.
phthalic acid reacts with resorcinol to give intense green orange flourescene called fluorescein dye. This is a test to detect ortha-dicarboxylic group.
 1
1Guys! I know it's discussed but can you confirm that CCl3CHO doesn't go cannizaro reaction.
 1
1@govind,pritish and ankur......
thanx for excellent response[1][1]
keep more of it coming...here[1][1]
 39
39Ankur you're right chloral undergoes HALOFORM reaction in basic medium instead...as halogen-substituted alpha carbon of aldehyde/ketone can respond to haloform reactions(if the case is like this).
Another exception to Cannizzaro is HC(triple bond)CH-CHO....it forms acetylene and formate anion instead.
 1
1Thanks for that Pritish; I didn't know that.
Next: Keratin, a structural protein is present in Skin, Hair, and wool.
PS: It was asked in TS, with multiple answers.
 1
1This one is not exception, but it's worth mentioning:
tertiary halides reacts with LiAlH4 to not-to-give alkanes; They give alkenes.
Similarly, primary halides do not react with NaBH4 at all.
To solve this trivia out, another reducing agent is used: TPH (Triphenyl tinhydride), it manages to convert every kind of alkyl halide to corresponding alkanes.
2) Boiling Point of alkanes VS Number of C atom is a linear curve, while Melting Point VS Number of C Atoms is zig-zag.. i.e, alkanes with even number of C atoms have higher mp then odd C atom chain.
 11
11Nice thread..[1]
Chloral is an important exception to Cannizzaro rxn.,
the final product is Cl3CH + HCOO-



