 1
1@Aveek: nothing co-relating. It's just Test-Series. :D
 29
29HCOOH..Formic Acid shows positive Tollen's and Fehling's test..
The attack of NO+ on benzene is faster than NO2+
In case of non-polar medium the formation of NO2+ is the slow step... and in case of polar solvents the removal of proton will be the slowest step in Nitration...
The vant hoff factor in Nitration mixture is ≈ 3.82
In case of Sulphonation the electrophile is SO3
 1
1esters having one α - hydrogen do not undergo claisen condensation reaction..........
however with strong bases such as triphenylmethyl sodium, some esters like ethyl isobutyrate undergo claisen condensation ..
 1
1not an exception but it's good to know these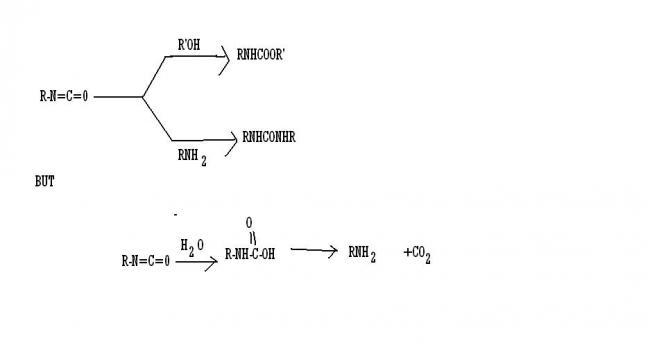 ----
----
 1
1[1][1][1]nice work friends[1][1][1].thanx to u all......
P.S.yes,abhishek.......no such thing tht u only need to post exceptions any such thing which is rare to find n notice+exceptions+other properties+more........caN BE ADDED[1]
 1
1@abhishek : for Claisen condensation : I didn't know that! But how can this be true. I mean, there is well defined reason why atleast 2 alpha hydrogen is required for Mr. Claisen... How will it attain equilibrium going with that fact..
 ----
----