The more feasible one would be the products in the first image you gave. In the benzyne mechanism, the resonance effect does not count due to the triple bond's presence. The inductive effect is responsible for stabilization of the carbanion formed on nucleophilic attack. The methoxy group has a -I effect and a +R effect(which is not considered).
The products in the first image are present in, I would say, 55%-45% ratio. This is because the methoxy group's inductive effect is weak, and the major product (due to more stable carbanion) is only slightly major.
What are the products obtained when meta bromo touluidene is treated with KNH2 in liquid NH3?
ans-
I have a confusion. Acc. to me it should depend on the structure given-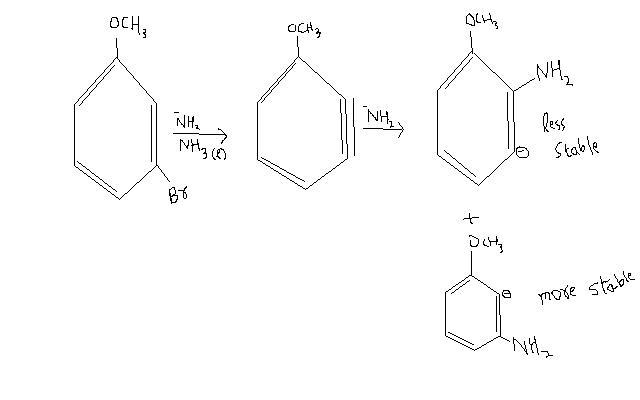
and
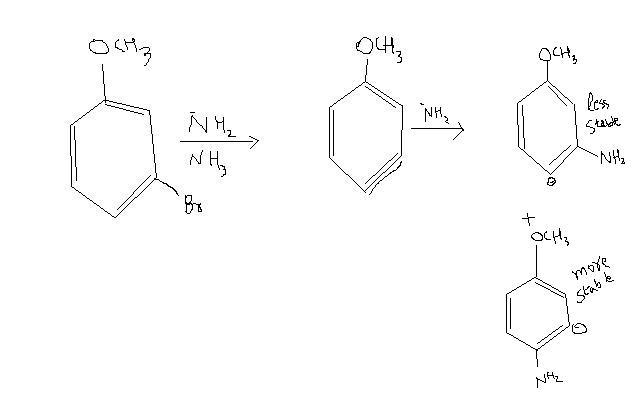
Which one is possible and why?
-
UP 0 DOWN 0 0 5

5 Answers
Haha....I trapped you Pritish.....look even I thought it should be first one.
But according to Jagadamba Singh Organic Chemistry,
The reaction occurs in both mechanisms, because the bromine atom has two alpha hydrogens down and up, so any one will sacrifice to form a double bond.
But the strange thing is that the para product is major one (where carbanion will be in meta position). According to me it should be meta product (where carbanion is in ortho position) because greater -I effect is there and more is the stability.
Please help me resolve this paradox.
Swordfish, I agree that both images are possible...I never said they aren't. I was talking in terms of feasibility..lol.
If you're referring to the second image where we have a meta and para product, of course the para product will be major. The m-carbanion is closer to the methoxy group and will be slightly better stabilized than its para counterpart. You're confusing the resonance and inductive effects. The resonance effect has a directive nature but the inductive effect only depends on the distance between the substituents.
This is why I said the first image products are more feasible, because the carbanion has a chance to be closer to the methoxy group. The second image products will be minor in comparison to the first.
And this is an anisole...not a toluidene :)
No I'm not getting confused between resonance and inductive effect.
You are saying that the reaction in first image is more feasible. In other words, meta product will be formed in major.
But two books- Brilliant Tutorials Chemistry and Jagadamba O.C. both say that para product will be formed in major. Can you explain why?
oh yeah its anisole....slip of tongue you see [3]
I don't really trust Indian books. Unless you can find a reliable source....I'll look it up tomorrow k? :)