ok comng up with 3D thing
Toluene is treated with Cl-CH-CH2-COCl
l
CH3
in the presence of anhydrous AlCl3 The product formed is
Here friedel craft acylation will dominate or acetylation
-
UP 0 DOWN 0 0 43

43 Answers
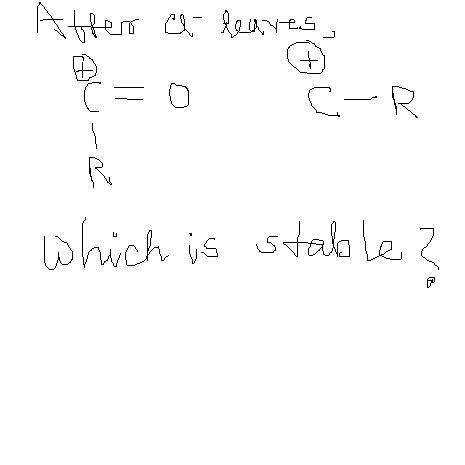
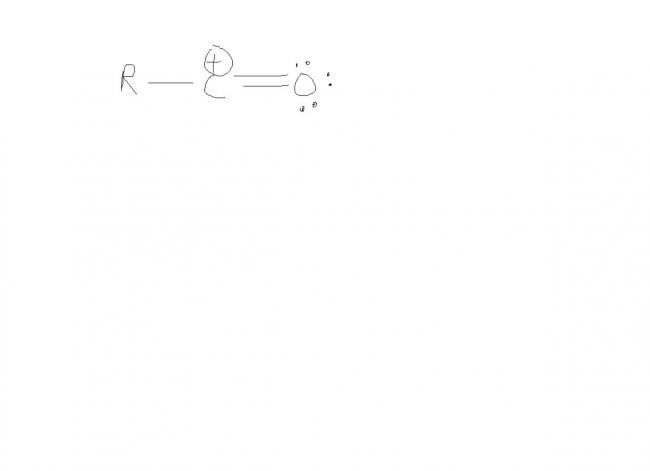
O has -I effect... it will mame the +ve charge more unstable..
thats y i told acylation is difficult.
nah think a bit more sky
later it becomes obvious that acyl one is more stable .
your structure gives one clue I think . well no I'll post one with clue. ok ?
am not getting any clue really...
donation of lone pair is possible only in case os alpha carbocation...
ring any bells ???????
if not check this
see . the O atom donates electron pair . so the carbocation is not there O gets +charge . but octet of every atom is complete here hence more stable .hope you got it . there's a previous JEE Q on this assertion reasoning. Think ull find it in OPA
no sky !!!!!!!!!!!
[343]
the first thing you suggested is impossible not second one .
kahaan padhi tumne ??
please forget that thing it wrong this I'm sure. [341] cut it off from your head . totally wrong . bura mat maan na . par please don't think of such a thing
no nothing to mind...
thanx.... to correct me...
well power cut...
am sorry :'(
u post i will see wen it comes..
no am back to square 1...
just got to know dat i am wrong ... but dunno why..........
sri i cant get ur structure
how come O has 10 electrons
or i m missing something [7][7]
sky girl   u are 100% correct in your approach  C+=O is highly unstable
sry vishal . there's one extra lone pair of electron.on O .
it should be this.
sry for mistake in figure.
here octet of every atom is complete so it's the major contributng structure.
I mean second structure.
woh 3-D wala me bhi confuse ho gaya isliye delete kardiya . sry ab jyada time nahi hai to baords ke baad post karta hoon . sry sky. :(
Please explain the reason for the ans and also in the ans it is according to alkylation first
OYE the other option shud b alkylation!!!!!!
ye dono mei wats da dif : acylation will dominate or acetylation