guyz plz try the conversions
1-3 dibromopropane ---->(Zn + Δ) A ------> (HBr) B ------> (alc. KOH) C ----------> (Br2) D ---------> (alc. KOH/NaNH2) E
identify A,B,C,D,E
-
UP 0 DOWN 0 1 31

31 Answers
I think ans is D.
cyclopropane(3 membered ring) is more strained(Baeyer strain theory) than cyclobutane(4 membered).
the last one is your bicyclic compound . not the other one .
b/w one Q
St.A : cyclopropane is formed more easily than cyclobutane
StB : the energy of activation for formation of cyclobutane is more
ok..
another one...
1-bromo, 3-chloro cyclobutane --->(Na/ether) ?
For any terminal alkyne
R--C ≡ CH ---NaNH2----> R--C ≡ C - --- + R' + (from R'Br )----> R--C ≡ C--R'
CH3--CH2--CH2--OH + H2SO4(conc.) ---> CH3--CH=CH2
----NBS----> CH2(Br)--CH=CH2 ---aq.KOH or noist Ag2O----> CH2(OH)--CH=CH2
In 1st Q.
2 moles of acetaldehyde shud be mentiond.
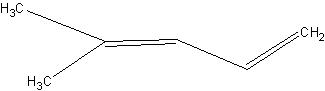
So final pdt has 7 C's (conjugated diene)
2nd is also a conj.diene.
bhaiyya......
no. of C's in 1st ans are 1 less.[1]Also H missing(But its understood).
er....rkrish I think u r wrong,,,,the Br shud b at the 3rd carbon....(allyl)
NBS reaction proceeds via free radical.
| |
--c=c--c-- + NBS ---> -c=c-- c --
| |
Br
name the alkene which on reductive ozonlysis gives the following products:
(i) acetaldehyde + 2-oxo propanal 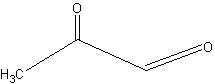
(ii) acetone + glyoxal + formaldehyde
1)
2) 
convert
(i) propyl alcohol to allyl alcohol (CH2=CH-CH2-OH)
(ii) ethyne to propyne
name the alkene which on reductive ozonlysis gives the following products:
(i) acetaldehyde + 2-oxo propanal
(ii) acetone + glyoxal + formaldehyde
see the important part to notice here is that the reaction is kinetically controlled and not thermodynamically .
the formation of cyclohexane is thermodynamically preferred but the afifinity for Br for Zn is high hence it abstracts th Br nearby and leaves as ZnBr2 .
hence cyclopropane is formed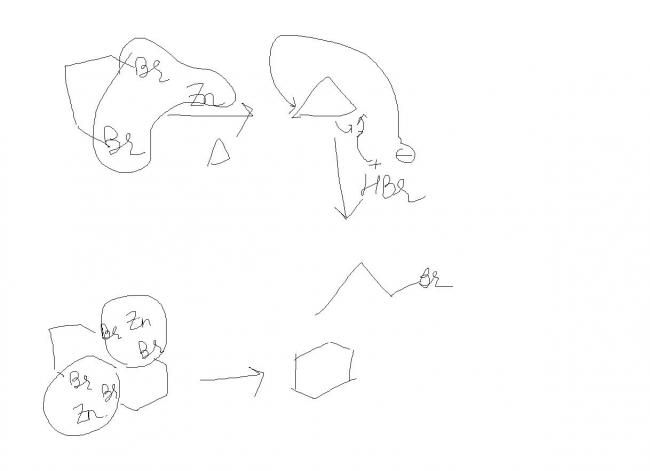

 √
√