39
39Now I remember...acid chloride and acid anhydride cannot give the haloform test due to the presence of electron withdrawing groups.
(Look through my notes)
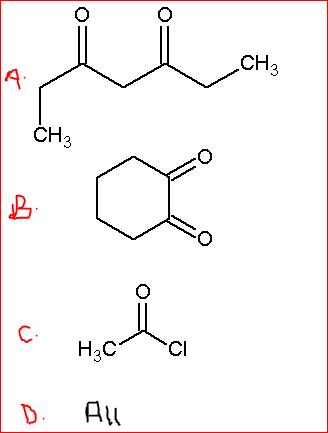
The answer is B, if those ketone groups are separated by one carbon atom and not as given in the figure.
 71
71I don't think it's correct!
And figure is correct as given!
 21
21I am confused
The electron density is decreased , so it cant donate electron to Br. i am fine.
But at the same time the -ve charge is stabilized by the -I effect , No ?
 39
39In that case is A the answer? Did I overlook something....perhaps an active methylene group?
@Shubhodip : Confused at which case?