In the First one the enol form is more stable than the keto form..because the ring attains aromaticity in enol form..
In the Second structure..the keto form is more stable..see this link for the reason
http://www.targetiit.com/iit-jee-forum/posts/why-2364.html
how tto find out which one among the tautomers is more or less predominating???
for example in pairs like as follows....
1.)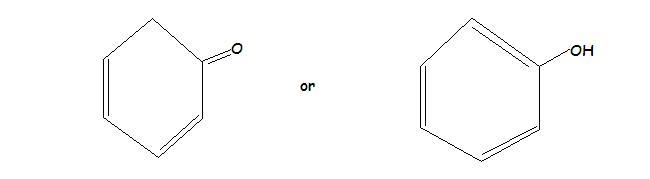
2.)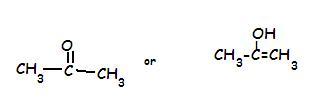
please give full working...neither do i know the method nor do i have the answers...[2][2][2][2]
-
UP 0 DOWN 0 0 2

2 Answers
govind
·2010-02-27 09:03:16