No....thats why I posted the ques here to get more info...and i really got a lot of info
anyways thanx everyone [1]
may be but i posted this soln. as a probable ans. Have to wait till eureka answers.
No....thats why I posted the ques here to get more info...and i really got a lot of info
anyways thanx everyone [1]
i think eure said that the answer is no product...
the mixture will get tarred....
http://www.arkat-usa.org/get-file/19548/
I think it's nitration at 2 position..
Manmay won't your case occur when heat is also mentioned?..
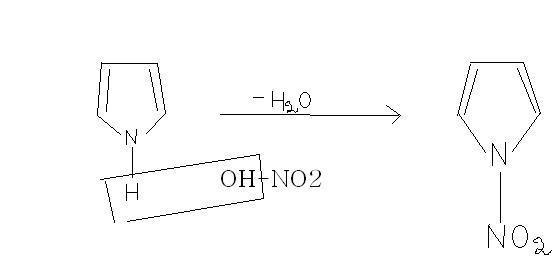 Is the ans like this eureka.
Is the ans like this eureka.
I think here nitration won't occur since there is no other reagent to give electrophile NO2+ and HNO3can't dissociate itself without any aqueous or other media.
A really old question........i dont even remember from where i posted it.....give me some time....i will reply on it.. [1]
Actually i was searching for the answer of this question on the net...didnt get any information abt this reaction..but got some other useful info regrading the reactions of pyrrole.......eureka plz tell the answer..
pyrrole with HNO2 forms N-Nitroso pyrrole..
However, pyrrole is unstable towards oxidising agents and therefore cannot be nitrated using the classical nitrating mixture of a mixture of concentrated nitric and sulfuric acids.
source : http://users.ox.ac.uk/~mwalter/web_05/year2/arom2/hetarom_rxn_mech.shtml
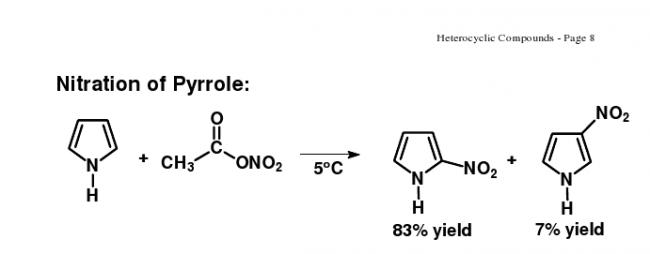
Source : http://docs.google.com/viewer?a=v&q=cache:dzm0RlyiaGgJ:chemistry.uah.edu/faculty/vogler/LectureNotes332/CH332Chapter26.pdf+pyrrole+%2B+HNO3&hl=en&gl=in&pid=bl&srcid=ADGEESgkYzLw3iv37H-wIxw5YXaFGk2okNVNh33qBlq7sQiFKrsmoBzvzgVYZ0uSyziuswKqEzZi9fpvH-AMNvWQwZgVt6BNmI6M0wKZHvf3Ye9fwJuTYWXMebhiSjTwklYKPvia4nvF&sig=AHIEtbRBq3YljNkuQqe240u1ZIw5c-U-TA
Actually ans is a bit surprising..surprised me too.....waiting for someone to help
This shows I have to do Amines topic as soon as possible. I'm not in position to answer this question.