so we compare the heat of hydrogenation with their stabilities and vice-versa right ??
What exactly is heat of hydrogenation ??Is it the amt of energy supplied during hydrogenation ??
Is this ok-- More the heat of hydrogenation,,less stable the alkene.
-
UP 0 DOWN 0 0 2

2 Answers
Samarth Kashyap
·2009-06-02 05:58:42
it is the amount of heat released when an alkene is hydrogenated.
more the heat of hydrogenation less the stability of the alkene.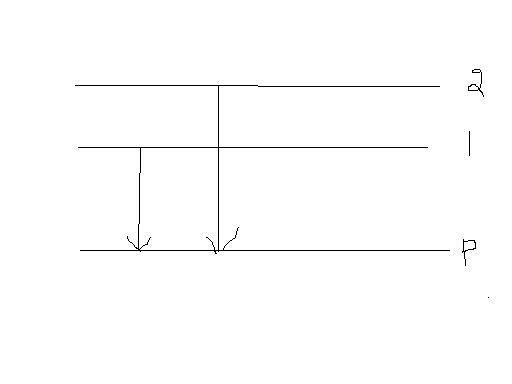
the diagram inicates the energy levels of the two isomers 1 and 2 and the final product P.
the second isomer has a greater heat of hydrogenation than the first.
from the diag. energy of the second greater than energy of the first.
so the second isomer is less stable than the first.
Gone..
·2009-06-02 06:03:35