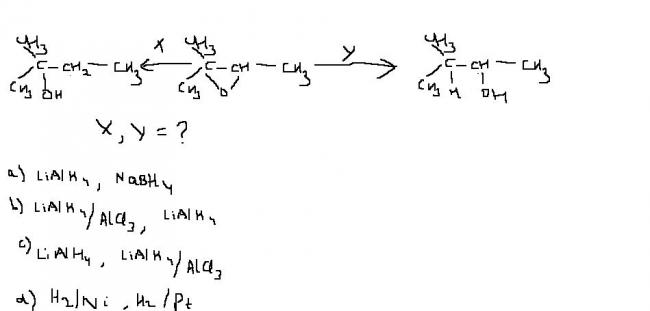NaBH4,LAH
7 Answers
the reason why i posted this one here..is becoz i wanted to know why hydrolysis of epoxides with LiAlH4 is acid type and with NaBH4 is base type????
(b) and (c) are not Possiblie because AlCl3 takes away theH2O molecule thus impartes Oxidising Character but in (a) and (c)
the (a) is correct because H2Pt cannot reduce properly
and it is correct that hydrolysis of epoxides with LiAlH4 is acid type
and with NaBH4 is base type
@ kartik I want to know why hydrolysis of epoxides with LiAlH4 is acid type and with NaBH4 is base type???????
http://www.chem.ucalgary.ca/courses/351/Carey/Ch16/ch16-6-2.html
hmm this mentions that hydrolysis with LiAlH4 is base type
i thought this another way.i don't know wht the answer says; it ws something which struck my mind.
LiAlH4 is a strong reducing agent , as all know. now, it cn cleave the O-C bond with more sterically hindered group i.e. the left side. and the cleavage occuring frm the less sterically hindered side would be minor product.
but the reverse is hsppening. why ?
answer is possiblity of pinacole-pinacolone reaction. ( OH on a 30- Carbon and + charge on a 20 carbon.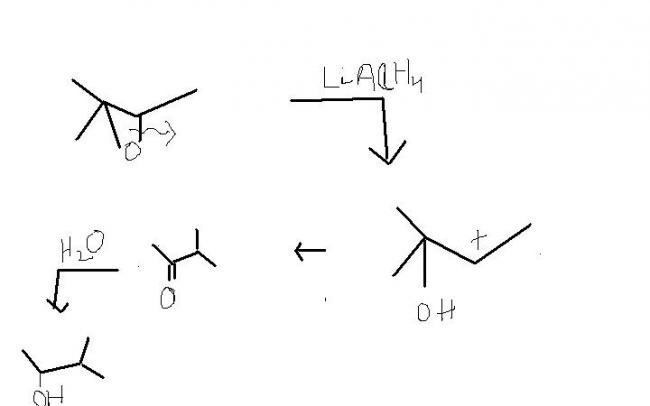
now , this property of LiAlH4 is destroyed by an acid as it reduces the its reducing nature. thus , AlCl3 acts as a lewis acid. and LiAlH4 / AlCl3 shows the formal method. ( cleavage frm more sterically hindered part)