b ..
1 just got 1 of dem from a frend.....so i m posting it here.........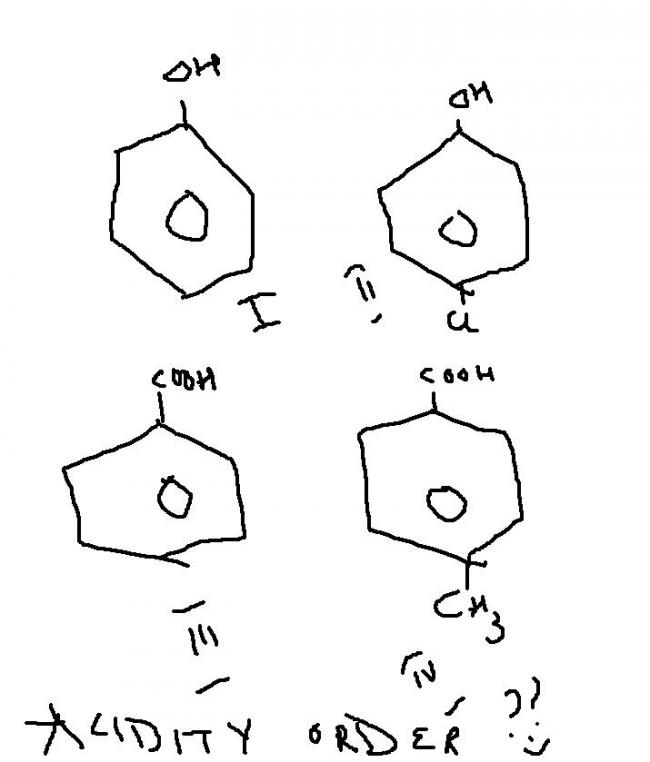
discuss d acidity order???
options...
a..2>3>4>1
b..3>4>2>1
c..3>2>1>4
d..4>3>1>2
i guess b is correct,,,whts ur opininon??
-
UP 0 DOWN 0 0 3

3 Answers
RAY
·2009-04-12 00:31:06
ok..here goes d reason..
2 is d correct options...coz for acidity we chek for stability of conjuagte base..
so after H is removed..
3 n 4 give equivalent canonical forms...which is highly stable....so 3,4 >1,2
now 4 has a methyl wid a +I effect from meta position which whill destabilise d carboanion,,,
so 3>4>2>1,,,
1 is a normal form..while 2 has -I effect that will help stabilise d carboanions...
hence..B is d answer...