Ans 4 ...
see (NH4)2S reduces one of the NO2 grps to NH2 then 2 Br attack at the ortho position of that NH2 grp....NaNO2 / HCl forms diazonium salt..Sn/Hcl reduces the nitro grp to NH2 then again Br attacks at the ortho position of NH2 ....see in this question temp is not mentioned so i am not sure whether N2Cl will be removed or not...if it's removed it creates a possibility that Br attacks at it's position but since the ring will be highly deactivated due to the presence of 4 Br grps so i dont think another Br will attack..so ans shud be 4 only
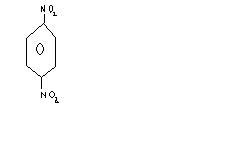 ----(NH4)2 Sx-----> A -----Br2/H2O ---->B ----NaNO2/Hcl---> C ----Sn/Hcl---> D ---Br2/H2O----> E
----(NH4)2 Sx-----> A -----Br2/H2O ---->B ----NaNO2/Hcl---> C ----Sn/Hcl---> D ---Br2/H2O----> E
Fond the total no of Br atoms present in (E) ???
-
UP 0 DOWN 0 0 4

4 Answers
govind
·2010-02-16 08:20:10
UTTARA
·2010-02-16 08:23:53
@Govind Can u draw E plzzzzzzzzzzzz?
So that it ill be easy to understand
govind
·2010-02-16 08:31:38
in E one position will be occupied by NH2 and the rest 5 by Br according to the answer....but i feel Ans shud be 4 as temperature is not given so it's not sure whether N2Cl leaves or not..and moreover the benzene ring will be highly deactivated by the substitution of 4Br that addition of another Br wont occur..