yeah, this can be formed. With simultaneous double abstraction of proton by base from ketone.. and attack on aldehyde's ring (the structure given is wrong, it must be phthalaldehyde] .
.
So the answer's process is like this: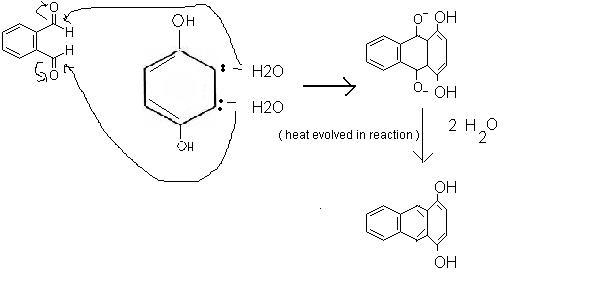 .
.
Hope it clears.
yeah, this can be formed. With simultaneous double abstraction of proton by base from ketone.. and attack on aldehyde's ring (the structure given is wrong, it must be phthalaldehyde] .
.
So the answer's process is like this: .
.
Hope it clears.
ohhh ......well it was a blunder....and no one else on this planet or even universe could commit that...
i thought this....
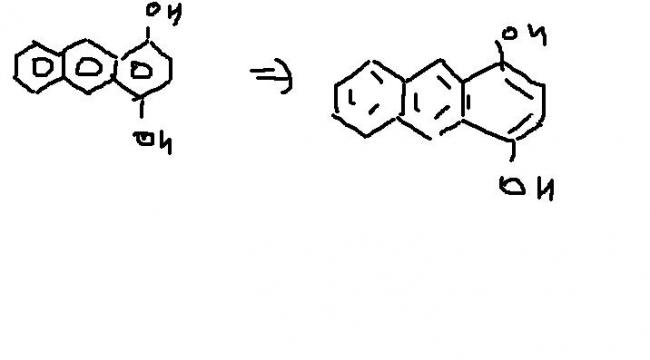
yeah that was really a terrible blunder.. :D ... and.. not good drawing in this case, when I mentioned you phthaldehyde, I searched it on net for it's structure so that I could have provided you.. and then used it in drawing.. .