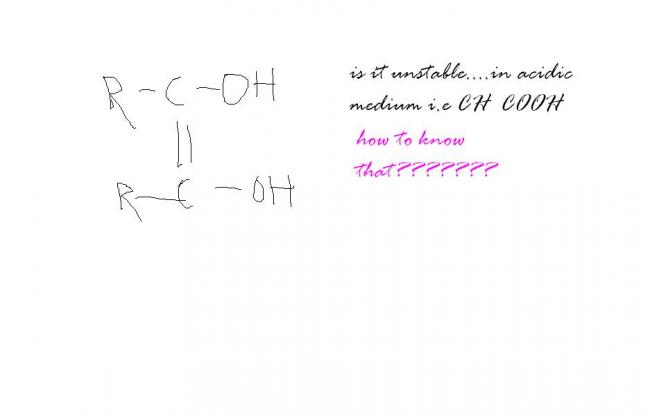2 Answers
Pritish Chakraborty
·2010-03-22 09:07:08
In acidic medium a proton adds to -OH and it leaves, forming a vinyl carbocation. That is extremely unstable. Hence it is unstable in acidic medium.
Tapas Gandhi
·2010-03-22 09:09:42
R=-CH3 is maleic acid
less stable than trans isomer
Maleic acid and fumaric acid can normally not be interconverted because rotation around a carbon carbon double bond is not energetically favourable.
However it can be isolated.