In 4), my guess is b). The keto group is electron withdrawing, and the carbon beta to the bromine atom becomes acidic, loses a proton by KOH. The carbanion formed removes the bromine atom by forming a pi bond with alpha carbon atom. Sort of like an E1CB mechanism. Primary halides are not so reactive to E1 and E2 as for both, stability orders are 3>2>1.
Some simple suitable ones for practice-
1) Which of the following cannot form Grignard Reagent ?
a) CH3CH2Br b) CH2=CHBr
c) HC≡CCH2Br d) CH2=CHCH2Br
2)CH3CH2Br ---(NH3)---> CH3CH2NH2 (Given 2b true), Hence..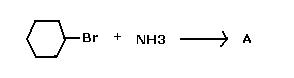
What is "A" ?

4)Most reactive towards Alco. KOH is --
a) CH2=CHBr b) CH3COCH2CH2Br
c) CH3CH2Br d) CH3CH2Cl
5)Arrange in CH3-X (X=F,Cl,I) in Decreasing order of Dipole Moments .
6) Identify Nucleophile in the following compound giving Intramolecular SN reac.n -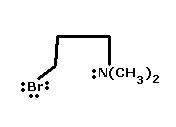
7) Select correct statement(s)-
a)Strongest force of attraction in alkyl halide is London Force.
b) London Force is a Surface Attraction.
c) Molecules with larger Surface Area have higher Boiling Point.
d)Perchloro Ethane is used as a Moth repellant.
(P.S. Some are my doubts; some are not [6])
-
UP 0 DOWN 0 1 55

55 Answers
@Qwerty.... Right fr the second, But how do v decide fr the basic action of NH3 ?
@Pritish.... 3,4 Correct, 6th mein N is given 2b the electrophile.
@Eure.... Not completely correct.
@Tush... A) is right fr 3).
Present Stats -
Q2, Q3, & Q4 Answered Correctly with answers - CycloHexene, a) & b) Respectively...[1]
becoz dipole moment=charge diff * separation
separationmethyl Fluoride<separationmethyl chloride
μmethyl Fluoride<μmethyl chloride
Wat sort of separation r v talking about here?...(mujhe sahi mein nahi pata[3])
The electronegativities of the halogens inc in the order I < Br < Cl < F
The carbon halogen bond lengths inc in the rder:- C - F < C- Cl < C- Br < C - I
@avik....hey in q2)....Sn2 to nahi hoga as the due to a large ring....there will be no backside attack....so Sn1 ya elimination...giving the temparature might be useful....else taking into account that NH3 is not that strong and since nothing is given let the temp be room temp. so the answer can found by be Sn1 reaction!!!!
in Q 2 major will be alkene , bcz NH3 will act here as a base rather than a Nucleophile
for Q2: NH3 acts as strong base as well as Nu but evidently, it's been seen that's it's Nuclephilic character is dominant in case of primary halides and in few cases (as you mentioned temperature) with sec. halides too.. but NH3 always surfaces it's base character with ring compounds or strained compounds.
Now there it goes.... Q7 -All correct !! (But why a &b?)
@Ankur...in Q3) Aren't they talking about Dehydrobromination ??
Q1) is not b) though..
@avik: Q3 is asking debromination isn't it? not dehydrobromination. That's why I came upto addition of halogens to alkenes.
debromination is basically anti elimination.. In (a) both Br's are cis wrt each other while in (b) they are trans.. so debromination can occur in (b)
Arihant-Arihant mein hi ghapla....!!
Meri mein toh a) diya hai...ek baar fir chk kar lijiyo, which one do you have ?