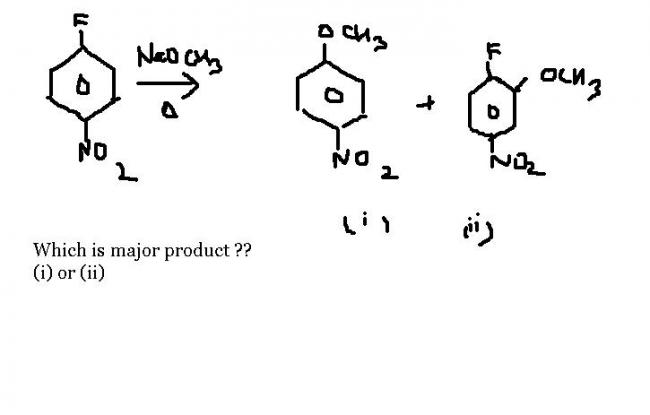
106
106ive sum dbts if the answer is (i)
my reasons:
(i) aryl halides do not undergo SN reaction due to a development of partial double bond between F and the aryl component and due to 3 other reasons given in NCERT
(ii) halogens are o-p directing, further -F is the least deactivating among the halo substituents
 106
106but maybe the ring is too much deactivated bcz of the NO2 and F group ... [7]
 24
24actually this is a really confusing ques....u cna find reasons favouring both
 1
1but how would deactivation result in the SN2 mechanism of 1) ? the position is para fr NO2 group.
 106
106deactivation se electrophilic substitution nahiin hogaa
i dont think it has any relation wid SN2
 24
24well looks like ans will be i) only
becoz SNAr is activated by EWG
and NO2 is a very goood EWG