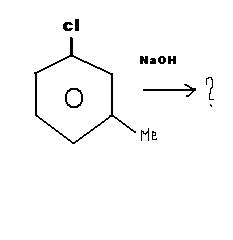
The product will be meta methyl Phenol in very extreme conditions..
Temperature > 623K and
Pressure > 300atm
7 Answers
govind
·2010-03-25 07:01:20
UTTARA
·2010-03-25 07:35:32
@Pritish : #2 Yes it is Benzyne Mechanism
#4 Dow's Process is something different i guess ??
UTTARA
·2010-03-25 07:36:59
@Govind Yes ur ans is correct
But can u show the mechanism ??
I mean the stability part
Y is m - cabanion more stable ??
Pritish Chakraborty
·2010-03-25 07:56:40
Uttara isi ko Dow's process kehte hain it follows benzyne mechanism. Only difference here is that there is a methyl group also.
Stability of m-carbanion is to do with inductive effect. Ortho position being closer feels the full brunt of methyl group's inductive effect...+R effects are not considered in benzyne mechanism.