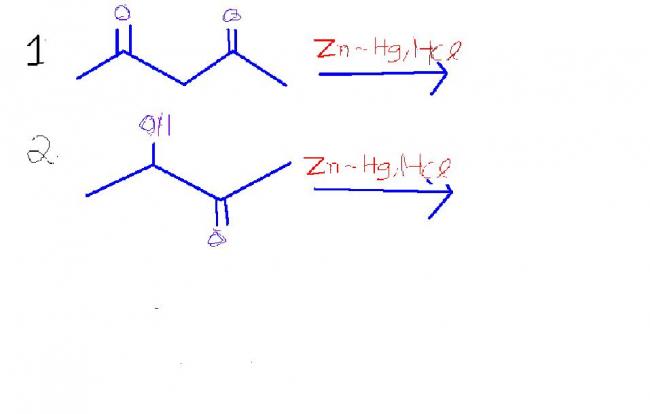
1) Pentan-2-one? One keto group is reduced.
2) Reduction is not successful. Substrate is not stable to strong acidic medium(medium reacts with -OH group). Carboxylic acids are not reduced in Clemmenson reduction, but their derivatives are.
8 Answers
Pritish Chakraborty
·2010-01-21 07:20:10
Pritish Chakraborty
·2010-01-21 07:22:04
If I give you an example mech of Clemmenson reduction will it be enough?
Check this out : http://www.organic-chemistry.org/namedreactions/clemmensen-reduction.shtm
rocky
·2010-01-21 07:23:33
why both cannot be reduced in (1)
(2) is not acid.........hint given for (2) is through hydrogenenolysis of OH group or olefins
Pritish Chakraborty
·2010-01-21 07:59:08
Then both groups could be reduced....if it specified one mole then only one group. I guess answer will be pentane then.