why no attack on pi bond
7 Answers
ch2n2 gives ch2- na. that is a carbanion. how can it attack on a pi bond?
Carbenes can be classified as nucleophilic, electrophilic, or ambiphilic. Reactivity is especially strongly influenced by substituents. For example, if a substituent is able to donate a pair of electrons, most likely carbene will not be electrophilic. Alkyl carbenes insert much more selectively than methylene, which does not differentiate between primary, secondary, and tertiary C-H bonds
Insertions are another common type of carbene reactions. The carbene basically interposes itself into an existing bond. The order of preference is commonly: 1. X-H bonds where X is not carbon 2. C-H bond 3. C-C bond. Insertions may or may not occur in single step
Intramolecular insertion reactions present new synthetic solutions. Generally, rigid structures favor such insertions to happen. When an intramolecular insertion is possible, no intermolecular insertions are seen.
thanks n also it will not attack pi bond as benzene ll lose aromaticity on attaching there
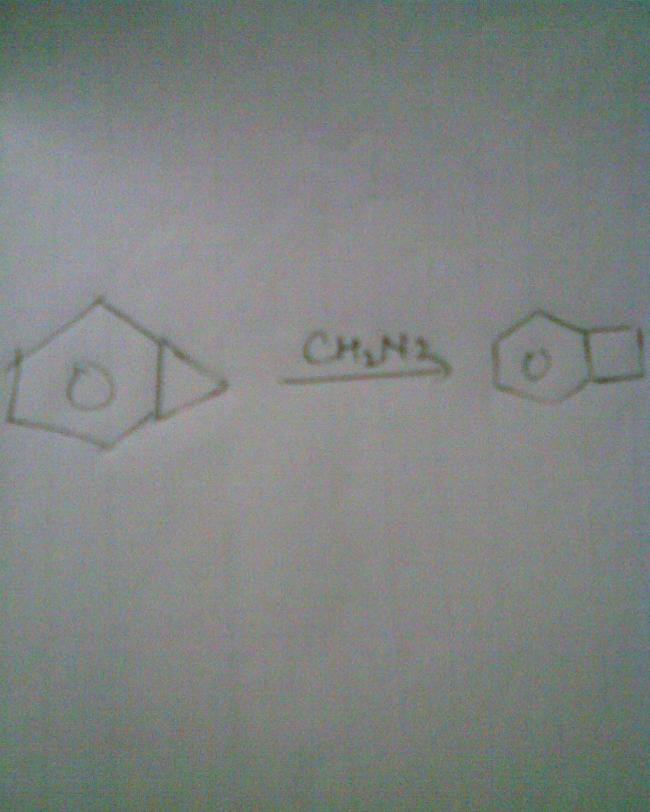 explain???????
explain???????