@iitimcomin...Dakin reaction is not in syllabus...[1]
so dont worry [1]
actually i diidnt know its name earlier..found it after some research..[1]
these all are not my dbts...just encountered tehse reactions here and there...I will continue posting more when I find them...
WARNING:These questions are a lot above regular Jee level...so don't try them if u r not good enuff with regular syllabus
1)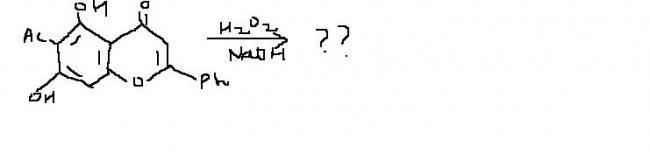
there is nothing special with DAKIN reaction.....just a kind of variant of BAEYER VILLIGER OXIDATION !
@iitimcomin...Dakin reaction is not in syllabus...[1]
so dont worry [1]
actually i diidnt know its name earlier..found it after some research..[1]
http://www.organic-chemistry.org/namedreactions/dakin-reaction.shtm
any links to mech of dakin rxn ...??? which chapter does it come under ..... is it in jee syllabus???
Dakin reaction follows Baeyer-Villiger oxidation mechanism...yeh toh batate hume babua! hume naam se ka matlab!
Trans-hydroxylation via epoxides? In that case a trans-diol will form at the place of that lone double bond on the right ring.
3rd question mein the reagent is hydroxylamine-o-sulfonic acid...dunno much about it! Can someone shed some light on these questions?
Ye 2nd question belong to which chapter eh?? 3rdis of amines and I've not done this topic yet.. quite lazy I'm
And in that DCC waala, peptide linkage hoga..on one side there is a substituted amide and on other side a carboxyl group. I think the ans will be a 5 membered ring.
Don't congratulate me pehle se hi...I need ans for confirmation.
Keep it up.. nicely done by Pritish. I couldn't manage to do that question.. and also the parts ahead..
Eureka.. good way.. keep posting such questions, I love these questions (no matter, I can't solve them :D ).
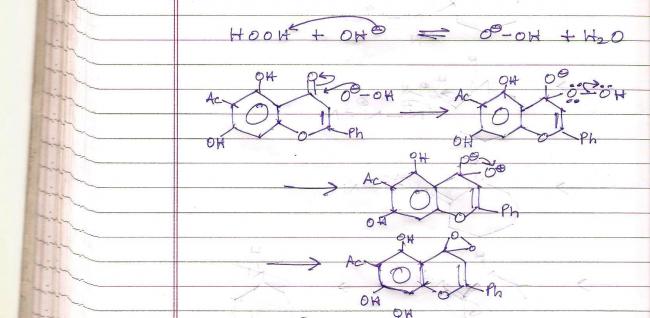
The hydroxyl group leaves due to strong lone-pair repulsion (Refer hydroboration-oxidation mechanism in which hydroperoxide ion attacks electron deficient boron center in a similar way).