yaar,H2 in case any catalyst is present it just convert -NO2 to -NH2.....
In the presence of ethanol....just let me think....or i'll try this tomorrow....i have practicals tomorrow....
yaar,H2 in case any catalyst is present it just convert -NO2 to -NH2.....
In the presence of ethanol....just let me think....or i'll try this tomorrow....i have practicals tomorrow....
There are only 3 ways to get aniline from NitroBenzene... And Pt catalyst is not one of those..
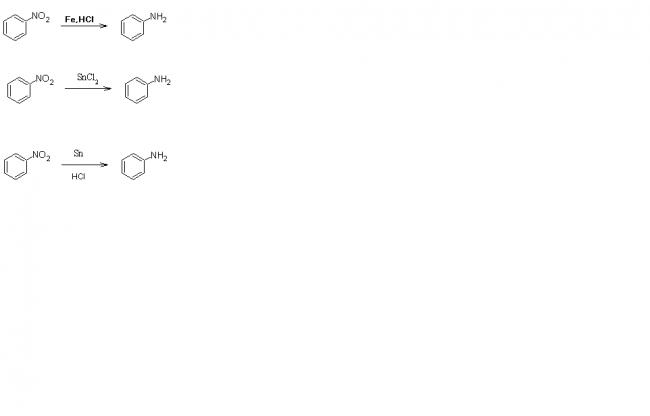
aniline will be the final product !
if that is what u are looking for !
coz intermmediates will be formed prior to its reaction with the base !
machan let the reactant be placed in pt metal da now wat will be the product
the product given by sankara is wrong...
The benzene doesnt want to lose its aromatic nature(very stable)..
So it wont convert double bonds to single bonds..
Thats why there should be some catalyst mentioned.....
reduction of this compound will give aniline for sure ...but u are just missing a catalyst and an intermmediate ... which will be formed prior to it reacts with the base