hey can u please post the image of the previous page of this post....
of the possible 2 attacks by the H+ ion which one is more favoured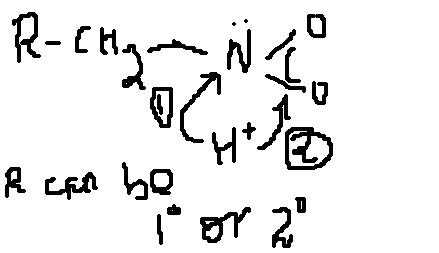
and explain why
-
UP 0 DOWN 0 0 26

26 Answers
In NO2, there is one cordination bond between N and one O.....that is obvious....
yaar, that positive and negative charge r due to the co-ordination bond formed between nitrogen and oxygen.....
does the positive charge on nitrogen develops due to transfer of lone pair of nitrogen to oxygen.....
but in NCERT the resonance structure of RCH2NO2 will have a partially positive charged nitrogen
@sankara,
hey yaar,
Since oxygen is more electronegative as compared to nitrogen therefore, the tendency of oxygen to give it's electron to H+ as compared to nitrogen will be less....
Therefore H+, acc. to me should follow the path 1.....
Also, since the electrons are delocalised in case of oxygen, therefore electron density at oxygen is less as compared to that of nitrogen.....
Also, due to +I effect of R-CH2 group the basicity of nitrogen is also increased....
yes prateek but these electrons are delocalised so i drew the diag like dat
hey buddy the second path u have mentioned is the attack of H+ on lone pair of oxygen or something else.....

