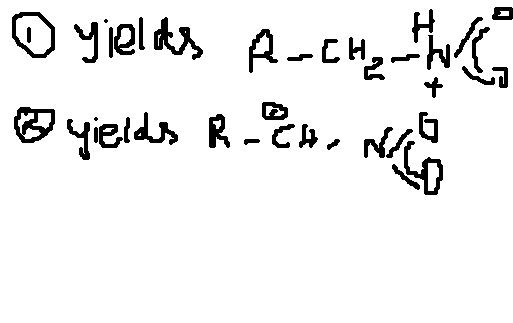[12]
12 Answers
second path is not followed..
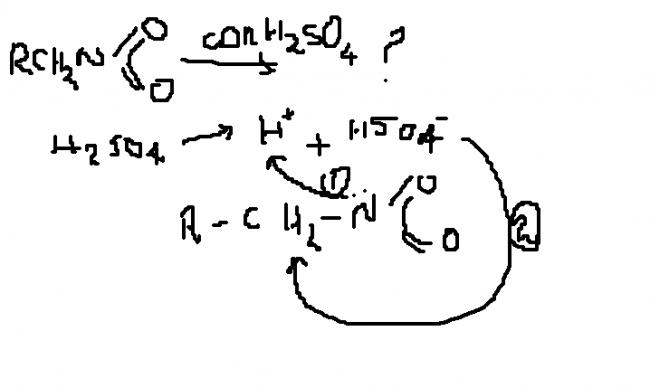
conc H2SO4 generates H+ which takes part generally...
conc.H2SO4 = very good acid.
=> HSO4- = weak conjugate base. => it is not so effective in abstracting H+.
so path1 is follwed...
yes skygirl i totally agree with u path 1 will be followed....
Also, the case can be as such since the -NO2 group is attached therefore +ve charge on -CH2- will be highly destabalised by the -I effect of -NO2 group....
same only re.. woh bhi H+ donate karega...
n the rest will be undissociated...
it wont matter...
yup buddy....
only rate will be reduced as Ka of dil. H2SO4 is less.....than Conc. H2SO4..if i'm right.....
txx for all u people for replying for my post i have just cleared abt the use of sulphuric acid
[3]
again i screwed up ...
that day i did this with carbon as well.. (had made it pentavalent in some question here in the forum only)
chhi chhi...
hey someone answer this one then....
thanx once agn srinath ...