is it (a) ?
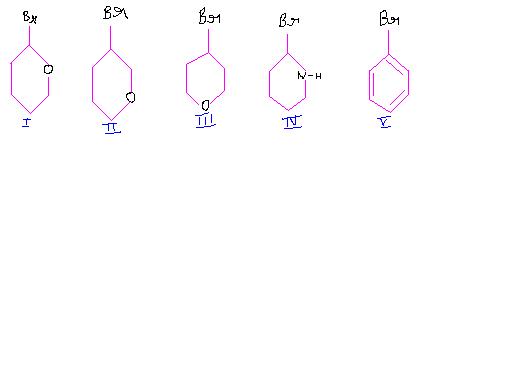
Ease of SN1 ractions among these compounds upon tratment with Aq NaOH will be in the order as:
(a) I >II >III>IV >V (b) IV>I>III>II>V
(c) I>IV>III>II>V (d) V> IV>III>II>I
-
UP 0 DOWN 0 0 3

3 Answers
Asish Mahapatra
·2009-10-26 04:16:45
ohh yes,
See, V will be least stable as it is phenyl carbocation.
I and IV have resonance possible so are most stable and as O is more electronegative than N, +ve charge on N is relatively more stable than that on O. So IV is more stable than I.
in III and II, O is EWG as it is electronegative, so farther the EWG, more is the stability of the +ve charge. So III>II
In I and IV resonance dominates inductive effect