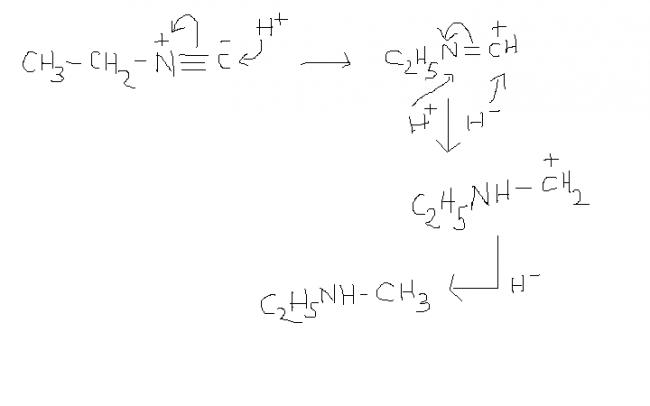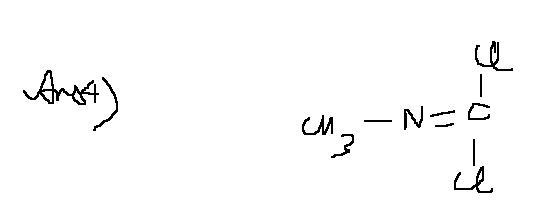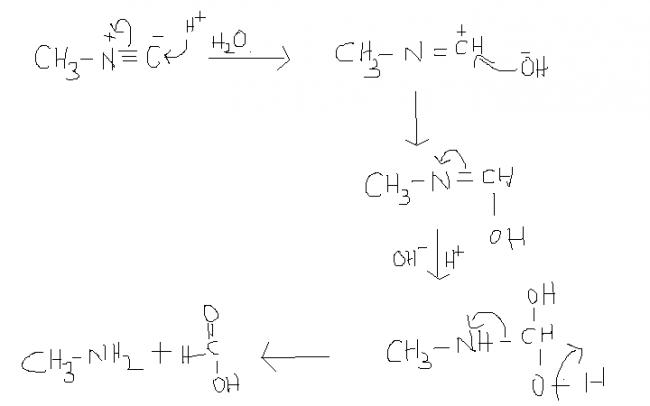1
11) i'm not very sure but this rkn should take place in the presence of a reagent furnishing H-. the given reagent only supplies H+.
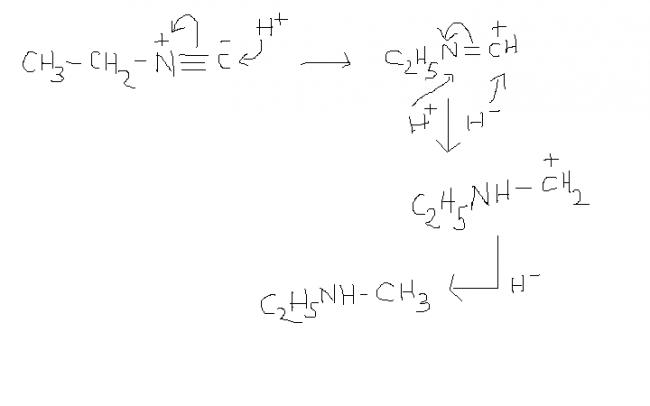
 1
1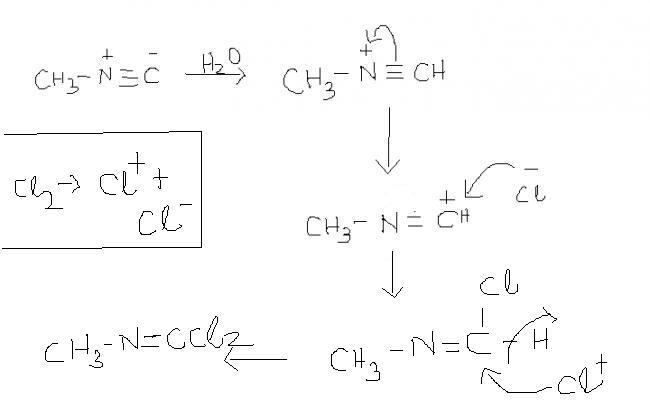
if more moles are used then, activity of Cl- will be more as ders possibilty of +ve charge on C , and also H+ also needn't be extracted out due to hindrance by two Cl groups.
also N- can't sustain another electronegative atom like Cl. thus, Cl+ would remain unreactive.
 1
1k. posting.just a minute.
 24
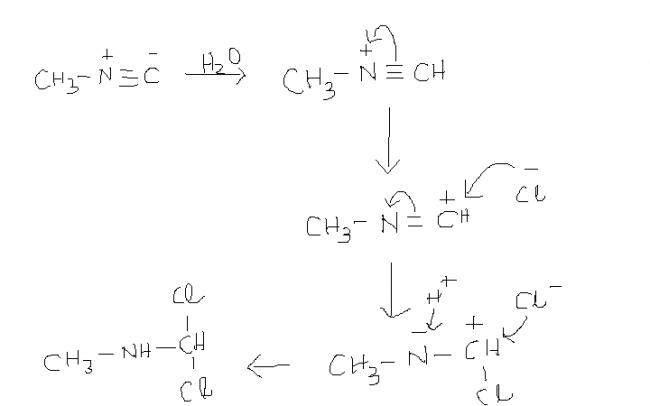
24so how will be mech in 4 then using 1 mol of Cl ??
 24
24hmmm.........let me think on it then..,,
anyways thanxxxxxx[1][1]
 1
1frm reagents like LiAlH4 or NaBH4 or NaH etc. anyone of reagents furnishing H- should hv been mentioned.
i strongly think so. but, if mechanism could be approached in any other way , then ?????
 24
24but from where will H- come ??
 1
14) ya, right. i hv taken two moles of Cl2 , dats why its coming so. otherwise the answer u hv given is right ( with a single mole of Cl2 ).
1) and 1st reaction should take place in the presence of H- , otherwise when H+ is added to C- in the substrate , the reaction proceeds in the backward direction and again the substrate is formed ( in the absence of H- )
 24
24Ans4 a bit wrong...here is the answer....
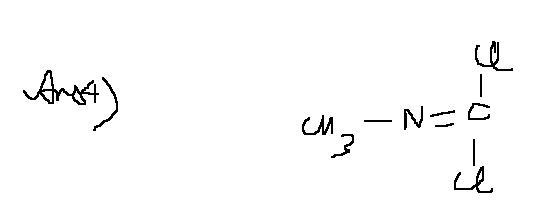
and one dbt in soln1 ......from where is that H- coming???
 1
14) this reaction takes place in aqueous solution.
 1
1help hs come.
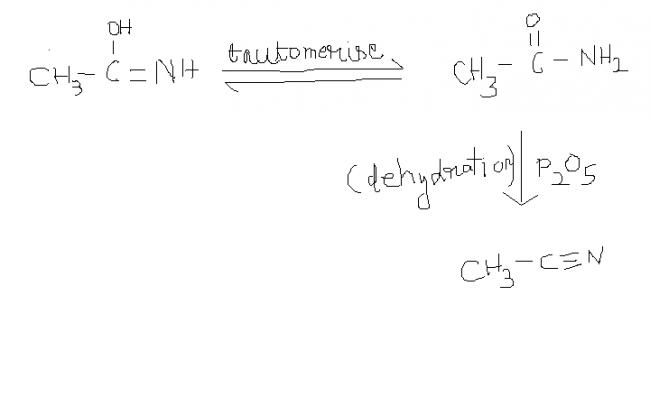
2) NOTE : keto form is more stable thn enol form. so tauto merism takes place.
 24
24hmm really ???
can u give the names ??
so that i can find easily ?
 24
24i want mechanisms....i have the answers
 4
42 ) CH3-N≡ C ----(acidic hydrolysis)----> CH3-NH2 + HCOOH (Formic acid).....this is also a test 2 distinguish b/w cyanides & isocyanides ...
 4
44 )CH3-N≡C + X2 ------> CH3-N=CX2 .... (X is a halogen).....
 4
41 ) R-N ≡ C + Na/alcohol -------> R-NH-CH3 ..... Na/alcohol is a reducing agent....