1357
1357For Question 1
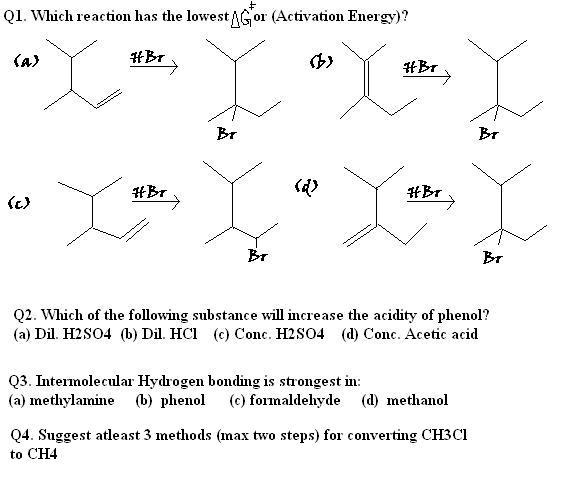
They are forming the same carbocation, So greater the energy of the reactant lower is the Activation Energy.
In B reactant is more stable than that of D. So difference of energy between the carbocation and reactant will be greater in B
 591
591Q1. (d)
Q2.Maybe (c)
Q3.I think (b)
Q4.(a)CH3Cl + Ni(300°C)→CH4
(b)CH3Cl + Zn-Cu couple/C2H5OH→ CH4
(c)RX + Red P/HI→ CH4
 481
481aur 1. ka i had doubt on (b) and (d)........in (d),there is a possibility of forming a 1 degree carbocation,which is les stable dan 3 degree,which increases d activation energy.......
But b mein both d possibilities are 3 degree carbocation.......so lowest A.E....
must have been (b),ans (d) diya hai.......Now think of an alternate method??
 21
21Q1) ka ans (b) hoga..... becoz 3 deg carbo are more stable and less act energy ....
i guess the ans may be wrong....
but can u plz tell me abt Q2)... i can't got it ..!!!
 481
481For 2) the only thing that is cuming to my mind is of the strenght of acid........Akshay tum kya logic lagaye ike liye????
 Akshay Ginodia Sry the answer to Q1 should be (b)
Upvote·0· Reply ·2013-12-11 04:38:22
Akshay Ginodia Sry the answer to Q1 should be (b)
Upvote·0· Reply ·2013-12-11 04:38:22 Anurag Ghosh 1.answer given is d
2.c...u r rite
3.b...again u r rite..but y not methanol,is it depended on more electron pushing group???
Anurag Ghosh 1.answer given is d
2.c...u r rite
3.b...again u r rite..but y not methanol,is it depended on more electron pushing group??? Akshay Ginodia in methanol the polarity of O-H bond is less due the +I effect of -CH3
Akshay Ginodia in methanol the polarity of O-H bond is less due the +I effect of -CH3