1.Ph-CHBr-CH2CH3
2.Ph-CBr=CH2
3.??in the propene branch,the ring is bonded to 1st or 2nd carbon???
 Dwijaraj Paul Chowdhury obviously 1st carbonUpvote·0· Reply ·2013-08-05 06:35:37
Dwijaraj Paul Chowdhury obviously 1st carbonUpvote·0· Reply ·2013-08-05 06:35:37
Rate of reaction-3>1>2
See the reaction will proceed with the formation of carbocation.......So SN1.....ab SN1 mein 3°>2°>1°...I hope this is d answer.....
1.Ph-CHBr-CH2CH3
2.Ph-CBr=CH2
3.??in the propene branch,the ring is bonded to 1st or 2nd carbon???
3) 1-chloro,1-propenyl ,cyclo hexane
Swarna ur answer is wrong....try again..
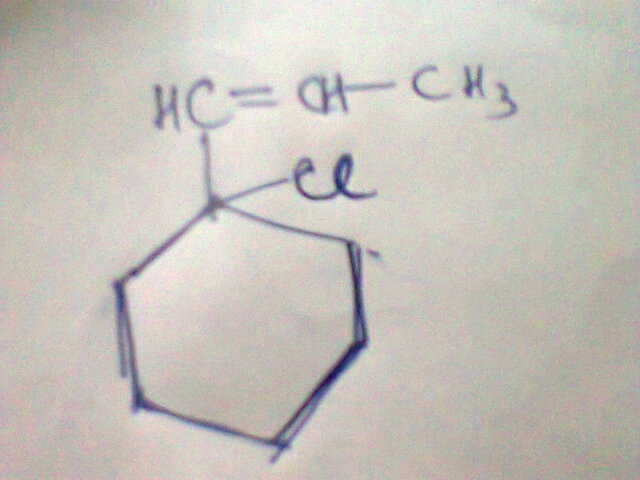
maybe ans wud be 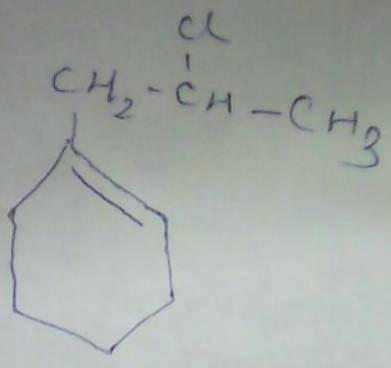
Hmmmm...Divyansh lagta hai tu r8 hai......didn't observed d pi bond inside...:P We r disturbing d aromaticity of d compound......
i just cant believe dat none of got it right......hehehe...cant stop laughing!!!!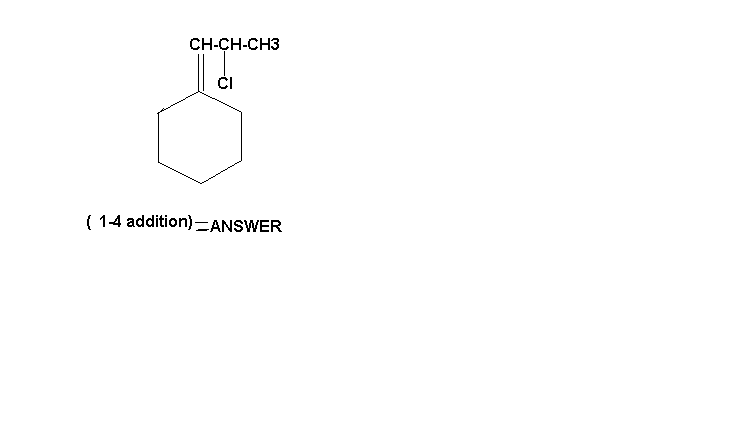
It is not aromatic all are not sp2 hybridized
Answer given by swarna seems correct to me