Why is cyclopropane forming from an alkene in Simmons Smith reaction?
Isn't dat increasing d strain...i mean isn't d product more unstable dan d reactant????
Heres wat i got for d reaction mechanism...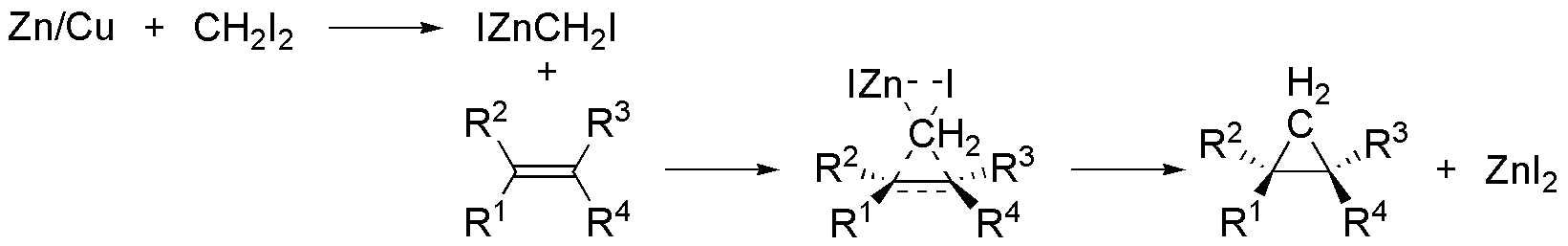
-
UP 0 DOWN 0 0 1

1 Answers
Vivek @ Born this Way
·2014-03-27 00:08:11
The for R groups on the alkene are under steric strain due to neighboring groups. Moreover, their constant inductive effect increases electron location on the -ene bond. This favors the addition of CH2I2 and also relaxes the steric strain by giving a near tetrahedral geometry at the two carbon atoms.
 Anurag Ghosh Bhaiya after so many days???..:)Upvote·0· Reply ·2014-03-27 01:45:38
Anurag Ghosh Bhaiya after so many days???..:)Upvote·0· Reply ·2014-03-27 01:45:38