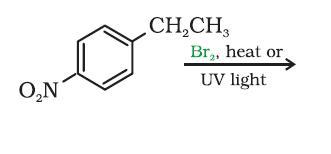
Bromine is a more selective and less reactive radical. It prefers secondary spots to primary spots. Here a benzyl radical can form, which bromine will love.
So attach bromine to CH2 carbon to get the major monohalo product.
11 Answers
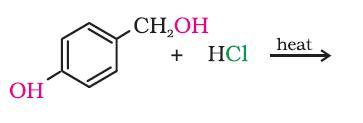
i guess it will be the secondary OH group....coz 2° more reactive than 1°....
verify!!
This reaction is pretty obvious subho. The -OH attached to the Ph group has partial double bond character. Hence it cannot be displaced so easily. We require either deactivating groups to be attached to the ring or stringent conditions.
Hence the CH2OH becomes CH2Cl.
nothing for me is obvious!! everything is an achievement in organic!!
Don't worry..you still have a year's time to polish organic. Even I was worthless in it till the end of 11th...in 12th it all changed :)
And I'll be uploading the rest of my notes sometime in May...so that'll help I hope!
http://www.targetiit.com/iit-jee-forum/posts/organic-moolah-15147.html