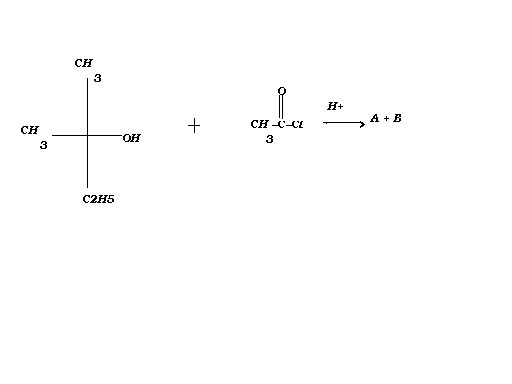
The two products formed here will be 2-ethyl-2-methyl ethyl chloride and acetic acid.
The chlorine atom donates one of its lone pair to the H+ , breaking the C-Cl bond. The C-OH bond breaks and the -OH attaches to the CH3CO+. The Chlorine atom attaches to the 3 degree carbocation as it is very stable due to +I effect.
2 Answers
Anirban Mukherjee
·2012-10-30 14:19:40
The questions says:
Complete the following reaction and give its mechanism in short.
My doubt is whether rather how will the reaction happen..?? As far as I know ,reaction of alcohols with acid chlorides takes place in presence of base catalyst!The hydrogen of the hydroxyl group is replaced by an acyl group (RCO-),resulting in the formation of esters.
But in the above reaction the question asks for the reaction to be carried out in acidic medium...
Can anyone give suitable mechanism for this with reasons?
Subhojit Paul
·2012-11-19 08:53:22
 ">
">