u said that the inductive effect dies with 4 carbons na ????
to now -I kahaan se aagaya ? for NO2 ???[7] the first one seems ok for me
ok..u only tell correct answer.......my ans. is one i posted in #11
well dude . see the -I of NO2 can be neglected .
the +I effect of C ofcourse has to b taken nito account
here the stabilising factor comes from resonace with NO2 grp
try writing the resonance structures is my advice u should get it
nope............u din get what i meant in 2nd reason........
i said NO2 HAS -I ON RING........RIGHT.ALSO,CH2 HAS +I EFFECT ON RING...............RIGHT!!!!!!!!!!SO, CLEARLY 2ND RADICAL WOULD BE VERY UNSTABLE...........HMMMMMMMM.
IN FIRST RADICAL TOO NO2 HAS -I ON RING.........BUT THIS TIME CH3 ON OTHER SIDE OF CARBON HAVING FREE ELECTRON WUD STABILIZE FREE RADICAL DUE TO +I EFFECT....................
DID U GET WAT I MEANT IN 2ND REASON.......
PLZ REPLY!!!!!!!
u said that the inductive effect dies with 4 carbons na ????
to now -I kahaan se aagaya ? for NO2 ???[7] the first one seems ok for me
no dear I'm sure :)
try writing resonance structures . u should be able to get it ya I'll post later
@ROHIT U R WRONG.................................................
SRINATH DA I CORRECT........
2 REASONS FOR THAT U SELECTED WRONG FREE RADICAL AS MORE STABLE........
1)STABILITY OF FREE RADICAL INCREASES AS PRIMARY<SECONDARY<TERTIARY!!!!!!!!!!!!!![3]
2)NO2 IS -I GROUP AND CH2 IS +I GROUP......SO SECOND FREE RADICAL U DREW WUD NOT BE STABILIZED(COMPARED TO FIRST ONE).....COZ IN FIRST ONE -I EFFECT OF NO2 WUD BE NULLIFIED(TO SOME EXTENT) DUE TO +I EFFECT OF CH3......MAKING FIRST FREE RADICAL MORE STABLE...........................................................
............SRINATH DA PLZ REPLY TO THIS ONE!!!!!!!!!!!!!
n can u clarify to me??how ill free radicals b stabilised by --R groups??i dun find any logic behhind it?????
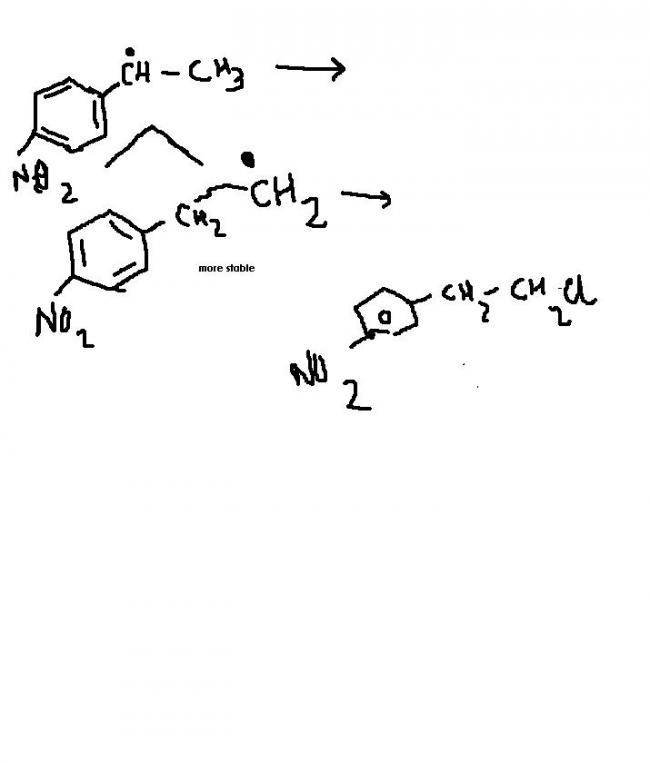
here if u name d above radical as 1...n d below one as 2...den in 1...wen there will b resonance due to ....--N+=0.....den der will b ∂+ charge at the ortho n para position...so dey will attract away electron from d free radicalln not donate electron to it...so d free radical 2 is much more stable......
see guys i understand dat the answer is becoause u wanna say by +I effect d free radical will b stabilised..
but srinath u r wrong brother..free radical r only stabilised by +i..or resonance..as dey have shortage of electron...so if d radical u guys r saying is forming..it will b de stablised by d del + forming on d carbon near it...due to d no2...hope u get it....
no.....no.nothing like that........you are like my big bro.........just assuring u!!!!!!!!
hey dude don't get me wrong or anythign . I was only stressing that you learn the cnocept that's all nothing more
. free radicals are stabilised both by +R and -R groups
will try to post more problems now don't have much time will certainly do so when I find some. :)
before.........actually i saw ur reply to relative reactivity thread.........n came here to ensur e whether i posted correct image or not!!!!!i saw u had not posted any comment till then n i posted wrong image.so instantly changed the image...just when the message of successful post appeared ....i saw ur point that ans. is wrong......................[1]...........don worry i won't ever cheat u or anyone else.........u can trust me that much[1]
wel well . that's right :)
when did u edit it ??when i told it was wrong or before ??[/hide
i added cl ...add br der....
c in second case...no2will have del + charge at para..so del + will attaract away e ...so make it unstable...

here if u name d above radical as 1...n d below one as 2...den in 1...wen there will b resonance due to ....--N+=0.....den der will b ∂+ charge at the ortho n para position...so dey will attract away electron from d free radicalln not donate electron to it...so d free radical 2 is much more stable......
OKAY........ONE MORE TIME.....LOOSE.......LOOSE CONTROL(RANG DE BASANTI)[1]
try dude . organic is fun . try it . only then will u remember longer. else if i give the asnwer u'll forget it quickly . generally this is what happens .
one clue
FR process
i don think it is right........but just thought so.............plz u only post the answer and explanation!!!!!!!!!!!