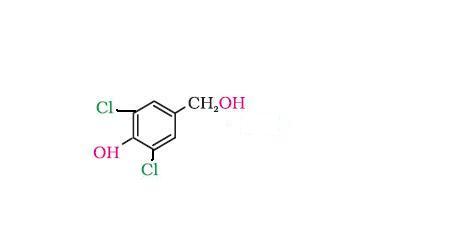
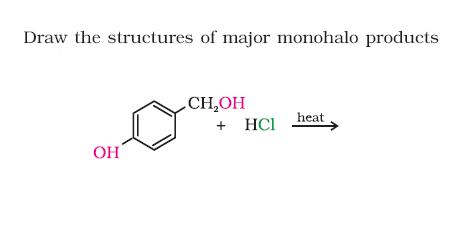
CH2OH group wld be transformed to CH2Cl
7 Answers
JOHNCENA IS BACK
·2009-05-01 22:49:56
REMEMBER IN ORGANIC CHEMISTRY XPLANATION MATTERS NOT THE ANSWER
gordo
·2009-05-02 03:01:09
H+ from the HCl protonates the OH attached to the CH2OH , OH2+ leaves as H2O...a carbocation developes on the CH2+ group, and Cl- attacks to give the CH2Cl....only if theres no wierd effect or some neighbouring group effect coming into play..., a very very minor product mite be the substitution of OH directly attached to the ring, by the Cl, but dats very rare, so u cud as well rule it out....
cheers!!
JOHNCENA IS BACK
·2009-05-03 02:56:54
hmmmmmmmm.......but don u think -OH being a activating group will resist side chain halogenation...n we will get this product