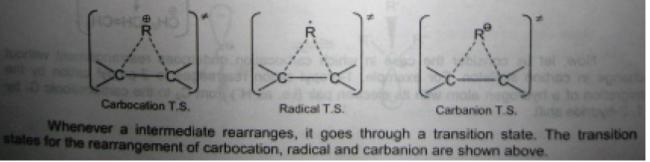
1
1u see when an intermediate is rearranging, the r jumps from one C to another to form a more stable intermediate
in this process, the bond wit the 1st C starts to break and a new bond wit the other carbon starts to form this is the transition state
wats the problem???
 3
3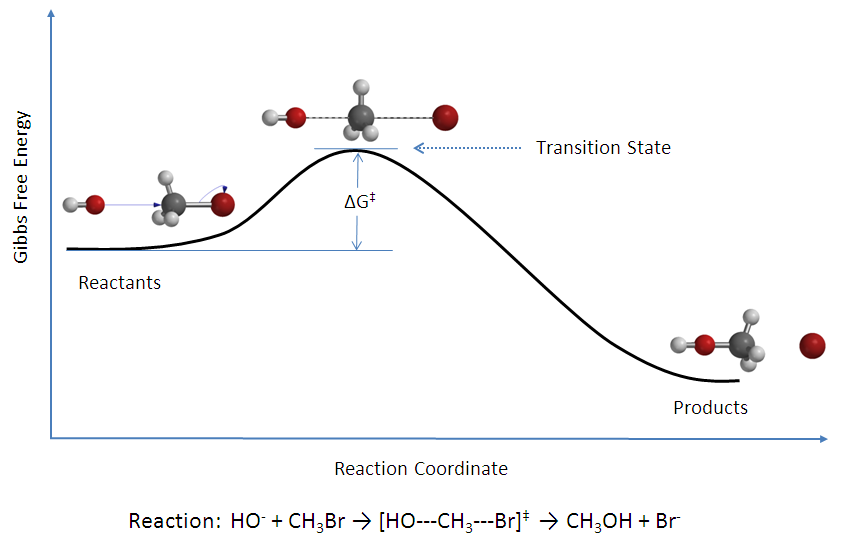
see the statement explains dat whenever a compound or intermediate rearranges it follows the general path as in the above figure
 3
3it means that the transition state will have the higher potential energies when compard to reactants and products.