have u got +2text john.......???........
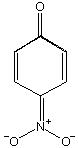
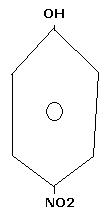
explain which is stronger acid among phenol and p-nitrophenol
-
UP 0 DOWN 0 0 14

14 Answers
well among phenol and p-nitophenol, p-nitrophenol is a better and strong acid: as the H associated with the OH group of the phenol is a bit acidic due to neighbor Oxygen then P-nitro group will easily form the H-Bond with that hydrogen and it will be very easy to be extracted and hence P-nitrophenol is a better acid!!!!!!
wel..........NO2 is electron withdrawing.......so d lone pair on O atom of OH is better delocalised.....so H can be esaily remove in para nitro phenol..........since H an be easily removed so its more acidic.....
[3][3][3][3][3][3][3][3][3]
@matrix n #2
nope!!! i m planning to get the +2 texts after writing aieee 2010
[3][3][3][3][3][3][3][3][3]
[3][3][3][3][3][3][3][3][3]
@akand
wow.....wat a quick reply.
wat the hell were u advicing me yesterday at 8:20 pm.cud u not post ur reply(sensible one) at that time!!!!!!
[3][3][3][3][3][3][3][3][3]
P-nitro group will easily form the H-Bond with that hydrogen and it will be very easy to be extracted [12][12][12]
how????????with this explanation u are explaining that p-nitrophenol will
be less acidic.
BTW there can't be no H-bonding here[4]
arrey tum ne electron withdrawing and electron reppeling groups ke baare mein pada hi hoga
uss ke basis par yeh ho jaaaega
@manipal
thanx...can u give an ans. in terms of resonance stabilisation(i.e.more no. of resonance structures)
i will say not exactly
it depends upon the position of NO2where it is placed!!!!
U will have to consider whether which group is attached
treatment will be different in case of CH3 and with NO2
So u have to be careful and apply ur concepts aptly
hey yaar due to the -R effect of -NO2 group there is development of +ve charge on the position of -OH group.....thus it reduces the electron density on the O-H bond and thus it helps increase the acidity of phenol....