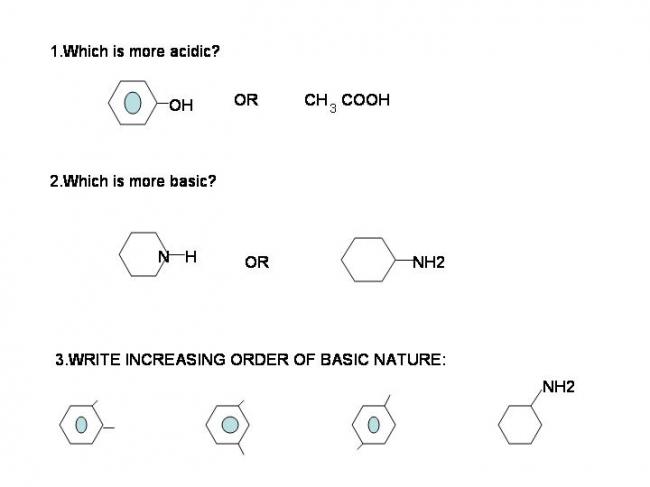
IN THE THIRD QUESTION,, one position contains CH3 and the other NH2...
-
UP 0 DOWN 0 0 13

13 Answers
NOW!!!!!!!!! C'MON YAAR... DUN GIVE ME JUST ANSWERS...
I WANT REASONS RE........ AM HOPELESS IN CHEM.. UNDERSTAND....
GIVE REASONS...
Y CH3COOH??
1) Because in CH3COOH, the CH3- Group is electron donating, and the difference in polarity of O-H bond is high .. but in the case of phenol, the polarity between O-H bond is not that high because the lone pair on O is in conjugation.
Therefore, it is easier for acetic acid to give away H+ and phenol
2) The lone pair on N in aniline is in conjugation with the Î -bond. But in the case of first compound, (not good at naming ;( ) N has the lone pair and can donate it , thus being more basic..
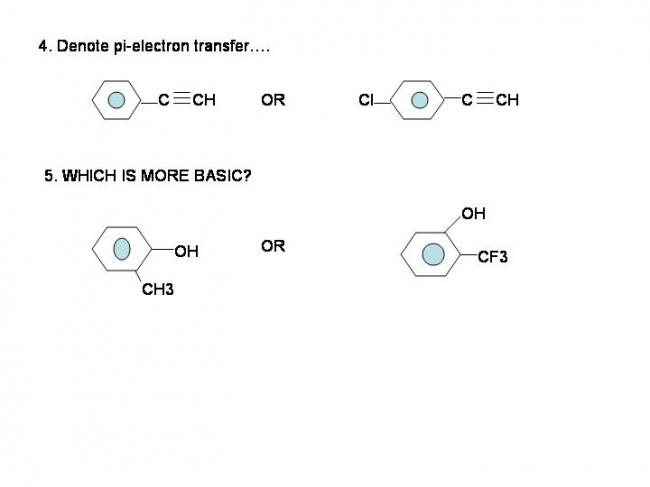
5) In the first compound, CH3- is electron donating while in the second compound, CF3- it is electron withdrawing, thus making O-H bond more polar and thus making it more acidic.
3) I think - meta < (ortho < para) < cyclohexanamine.
Cause, NH2 is a o- p- directing group and if CH3 is at meta position, it won't hinder the conjugation much and the lone pair is not available for donation.
check the 3rd and 4th Q's , I thinkk there are some NH2 gps missing.
in the fourth , is it Donate?
for d last q i feel d second compd is more basic reason being dat -ve charge on O will d stabilized by F
correct me if m wrng!
-ve charge on O will be stabilized by F
I don't get it .,.
F will withdraw the electrons and thus make it less basic ..
d base wich wud b more stble wud b basic na?
fisrt one CH3 has +i effct so it will increses d -ve chrge on oxygen n make it unstble weras F will hav -i effct n will widrwn or say delocalise d elctrons n make it more stable
(may b i m wrng)
More basic => Ability to give electrons. If F delocalises the electrons, then the electrons won't be available for donation right ?
@ integrations , the your reason seems alright but it's for the stability of the conjugate base of the give acid, as it's more stable , the second one is more acidic , hope you got it.
for d fisrt one simple reason is dat phenoxide ion is more basic so .conjugate acid is less
I think phenoxide ion is less basic than acetate. Phenoxide has -ve charge over C in the resonance structures while in acetate, it is over O...
no , in phenoxide all struc are diff , but in AcOH , they both are same. hope u got it.