I thought aldol condensation would give an unsaturated compound.. Dunno my answers not matching.
A compound C7H12 undergoes Ozonolysis to form B which on aldol condensation gives 1-acetylcyclopentane. What are the structures of the compds involved?
-
UP 0 DOWN 0 0 7

7 Answers
It's not possible. On aldol condensation, you must get either B hydroxy aldehyde/ketone or unsaturated aldehyde/ketone. Even looking from all directions, I couldnt' find that there is scope of ring formation here.
I think the final product we are talking about is 1-acetylcyclopentene
arrey, this is the same thing naa.. I'm saying, after ozonolysis we must get ketone coz it seems from further step. And, she is asking if on aldol condensation of ozonylated compound, we get acetyl cyclopentane: 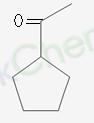 Now this only looks the case of intermolecular aldol condensation. And I am finding myself unable to do it. :(
Now this only looks the case of intermolecular aldol condensation. And I am finding myself unable to do it. :(