Yes, govind is right. Remember that one of the main steps to produce the aldehyde is hydrolysis of the gem-dihalide. Halides of the form >C-X2 are called gem-dihalides and on hydrolysis give aldehydes or ketones. This is because >C-(OH)2 is unstable and leads to >C=O instead.
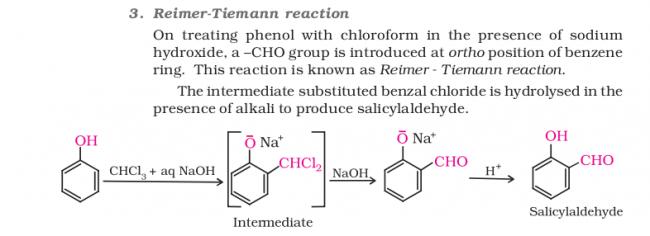
PARAGRAPH–Riemer-Tiemann reaction introduces an aldehyde group on to the aromatic ring of phenol,ortho to the hydroxyl group. This reaction involves electrophilic aromatic substitution. This is a general
method for the synthesis of substituted salicyldehydes as depicted below.

1.Which of the following reagents is used in the above reaction?
(A) aq. NaOH + CH3Cl (B) aq. NaOH + CH2Cl2 (C) aq. NaOH + CHCl3 (D) aq. NaOH + CCl4
2. The electrophile in this reaction
(A) : CHCl (B) +CHCl2 (C) : CCl2 (D) :CCl3
3. The structures of the intermediate I is?


-
UP 0 DOWN 0 0 3

3 Answers
Pritish Chakraborty
·2010-02-08 01:04:30