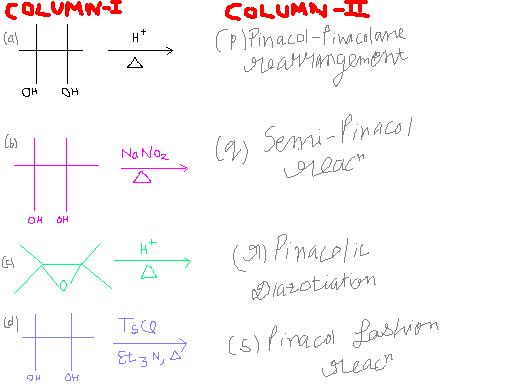
(a) -> (p) [Surely]
(c) -> (s) [3 membered rings do not give absolute pinacol reaction]
(b) -> (r) [NaNO2]
(d) -> (q) [Last option left? :P]
6 Answers
Correct Pritish
But please explain me abt Pinacole diazotitation and Pinacol fashion reacn.
For pinacole fashion reaction, in that 3 membered ring there is an O atom, so sorry I didn't notice that. Cyclopropane rings ka alag hota hai, they break in presence of acidic medium so pinacole reaction doesn't take place.
Here however the proton attacks the O atom, and one C-O bond cleaves away, forming a carbocation from where it is cleaved. Then a group migrates from the carbon which now has the -OH group, to the carbocation, as the lone pair of O forms a pi bond with the carbon atom. The protonated C=O+H then gives away the proton. Note that carbocation rearrangements/migrations occur even from three degree to two degree. Heat causes this rearrangement in carbon skeleton.
In pinacole diazotization, it is actually one -OH group and one -NH2 group, I think you may have written that incorrectly. Due to HNO2, a diazonium salt forms, which leaves and generates a carbocation by leaving. Compare this to usual pinacol rearrangements, where protonated water molecules leave first. After N2+ leaves, the usual process of migration and pi bond formation takes place.