I'm starting a thread for the resolution of many popular organic doubts which have remained unsolved in this forum. Two of them I managed to solve, for which I provide the links here -:
http://targetiit.com/iit-jee-forum/posts/can-any-one-do-organic-288-14473.html
http://targetiit.com/iit-jee-forum/posts/mechano-11811.html
Anyone who's organic doubt threads remained unsolved can post their links here. I have a list of many such older threads which govind had the courtesy to email me. Post more links of unsolved doubts here and we'll all try to solve them.
I shall start in the next post with the Clemmensen reduction of 1,3-diketones.
-
UP 0 DOWN 0 0 1

1 Answers
Clemmensen Reduction of 1,3-Diketones : Not the usual stuff
I take pentan-2,4-dione as my example here. Pentan-2,4-dione has the following structure, which some of you might remember from older threads on the same question/topic -:
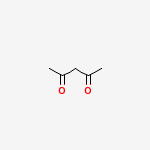
Often people have wondered how would the reaction proceed, myself included. Our first answer is usually that both ketone groups would be reduced. But the final product(obtained in high yields) points to a different mechanism when such diketone groups are present. Aakhri mein answer pentane ke jagah 3-methylbutan-2-one hai. Surprising?
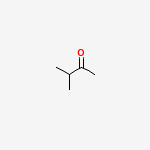
Notice how the main carbon chain has reduced by 1. Obviously there has been a rearrangement of some sort...and if you know the regular Clemmensen reduction mechanism, it will be helpful.
I did a little research on the topic and found this - http://pubs.acs.org/doi/pdf/10.1021/jo01017a512.
I'll give you the mech diagram right here -:
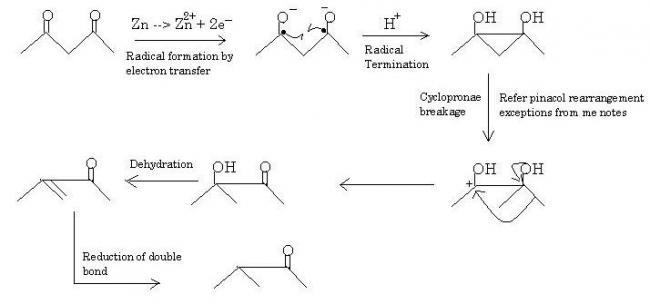
Pinacol rearrangement onwards, the procession of reaction is quite fast and the final product is obtained in high yield. Strangely zinc amalgam does not act on C=O but readily finishes off the double bond in the end. This is possibly due to enolation of some sort.
Note 1 : I could've tautomerized straightaway in the first step of pinacol rearrangement, it would give the same product on dehydration-reduction. But that's not exactly what happens, rearrangement does occur. Asymmetrical substrates will make it more clear to you.
Note 2 : If someone has a more appropriate mechanism, I'll gladly take it.