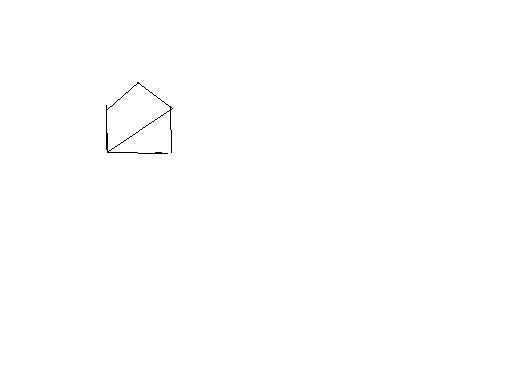
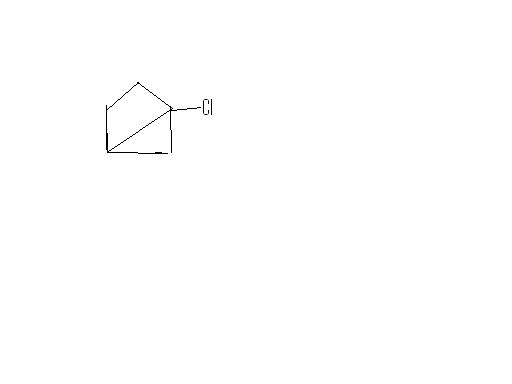
pritish u mean secondary spots (2o carbons) ?? i thought since this is free radical reaction,,it shud prefer 3o carbons,of which they are 2...but ans given is only one,,n im not sure how to decide.
11 Answers
Chlorine is more reactive, less selective so it attacks primary spots, of which there are 3 in the compound..
Relative reactivity of hydrogens towards chlorination
is 1-for primary hydrogen
3.8-for secondary
5 for tertiary
here we have 6 secondary so reactivity is 6x3.8
and we have 2 tertiary so reactivity is 5x2
so the product obtained by the substitution of a secondary hydrogen is the major for chlorination.
@sanky..
do we have to remember all thses values ??
1-for primary hydrogen
3.8-for secondary
5 for tertiary
Theek hai naa....Since the reaction would proceed via Free radical Mechanism, a Tertiary Hydrogen would be replaced by Chlorine....
Actually, the Extent of chlorination would depend on our external regulation.....so usually if Cl2/hv is mentioned we mention the 1st step product only (in most of the Qns tht i've seen)
If it were in a little excess, both the tertiary positions would get Chlorinated.....& the process continues...
but the preference for halogenation is just said.
for 1 degree: 1
for 2 degree: 3.8
for 3 degree: 5
preference for 2 degree: 6*3.8 = 22.8
preference for 3 degree 2 * 5 = 10
percentage for 2 degree H : 69.51
percentage for 3 degree H : 30.49
So alkyl halide with sec H replaced: 69.51 %
with tert H replaced: 30.49 %
dominant: secendary
Pata nahi..... v haven't been told these values....
But then what wud v have done it if were the case of Benzylic/Allylic free radical ?
This is the only case with halogenation of alkanes, not for other kind of substitution(as you mentioned, benzylic or allylic). These values are correct.
Wiki says:
* It is possible to predict the product distribution of different monochloro derivatives resulting from the chlorination of an alkane with non-equivalent hydrogens [1][2]. From experimentation it has been determined that the relative rates of chlorination to primary, secondary, and tertiary positions are 1: 3.8: and 5, respectively (this ratio is used in the example following this paragraph). This corresponds with alkyl carbanion stability: tertiary carbanion species are more stable than secondary carbanion species, and secondary carbanion species are more stable than primary carbanion species--thus any single chlorination will favor substitution at the most substituted carbon. Because of this trend, the percentages of each product formed from the parent carbanion can be estimated with relatively high accuracy. For example, 2-methyl butane ((CH3)2CHCH2CH3) exhibits the following results:
-For clarity, the unique hydrogens will be labeled as follows:
a=(CH3)2 (primary), b=CH (tertiary), c=CH2 (secondary), d=CH3 (also primary)
-Applying the aforementioned substitution ratios in the formula: ([number of hydrogens]*[ratio factor])/[(primary hydrogens*1) + (secondary hydrogens*3.8) + (tertiary hydrogens*5)]
a:6 X 1 = 6 a= 6/21.4 = 28%
b:1 X 5 = 5 b= 5/21.4 = 23%
c:2 X 3.7 = 7.4 c= 7.4/21.4 = 35%
d:3 X 1 = 3 d= 3/21.4 = 14%
Denominator total for this species = 6 + 5 + 7.4 + 3 = 21.4
Well..then how is it that in propane, chlorination of the primary spot is minor(45%) and the secondary spot is major(55%), while chlorination of 2-methylpropane gives primary major(63%) and tertiary minor(37%)?