thnx to all for explaining....
what will be the product when phenol is treated with nitrous acid??..
Plz tell the mechanism also..
-
UP 0 DOWN 0 0 17

17 Answers
Aromaticity brings a greater deal of stability than tautomerism. That is why phenols do not tautomerise.
I think YG files is wrong then..
becoz when it comes to assertion reason..they should ask only major product....
becoz if we start considering minor products..then there wont be just 2..there would be 1000 more products..based on probablity of reactants colliding...
it is formed no doubt but its percentage is very less.
This is because tautomerism is a case of equilibrium so either form will exist in some quantity.
The major one will of course be the aromatic product
this was a question in the Brilliant YG File question book.
It was an assertion-reason question.
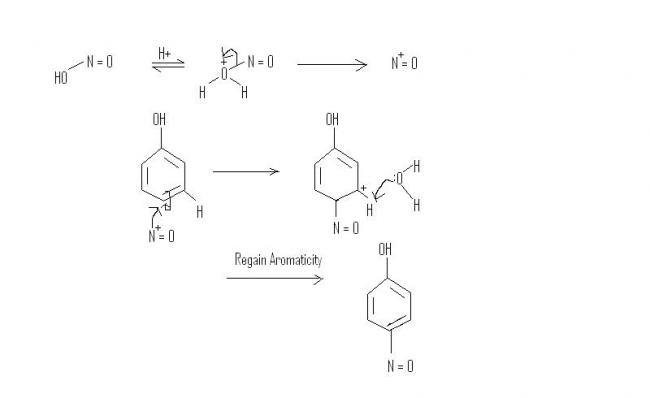
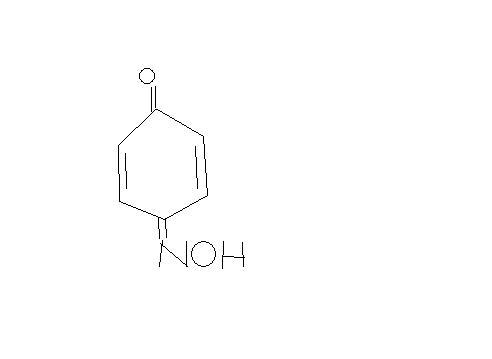
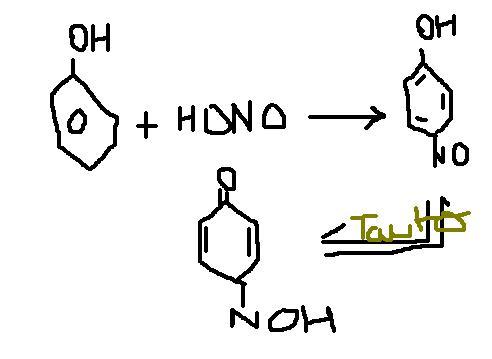
Statement 1- when phenol is treated with nitrous acid, p-benzoquinone mono oxime ( product shown by iota), is formed.
this statement 1 is given as true. so i was confused.
plz someone confirm the mechanism.
The statement 2 of this question was false so i've not given it.
Don't worry, even at FIITJEE most of the faculty is dumb and they will give the wrong answers to questions in phase tests at the expense of whether the student will get the proper knowledge or not. Our chem teacher(who has had many run-ins with the faculty) told us so. He has never lied to us.
Don't lose heart because they gave you negative marks. You know whether you're right or wrong.
But I got negative marks when I stopped the rxn at the 1st step...this question came in my test.!!! LOL I dunno y will it lose its aromaticity, but i think it will tautomerise...will confirm and tell tomorrow.
so question should be ...
which of these brings more stablity ??
aromaticity or tautomerism..
iota, why would it tautomerise and lose its aromaticity?
And for the mechanism, add a proton to OH in HO-N=O, water will leave and the nitrosonium ion(N+=O) will attack the benzene ring.