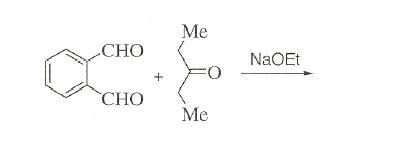
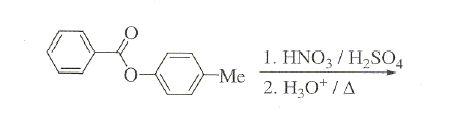
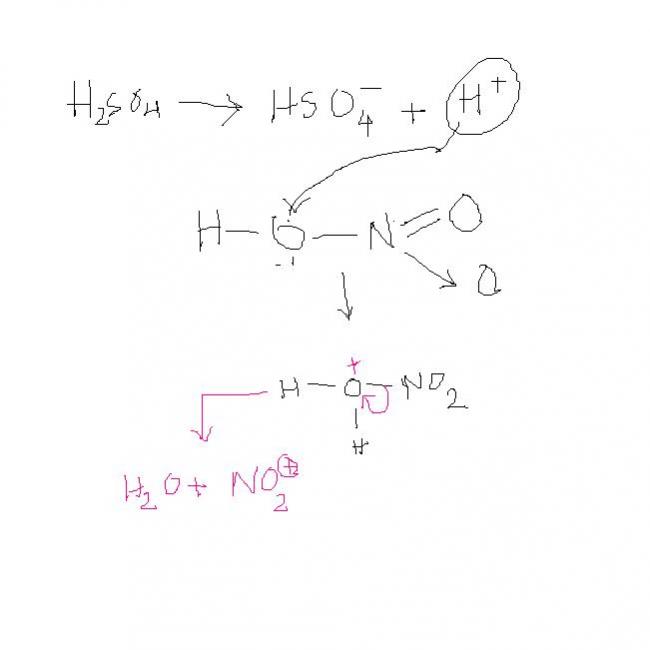
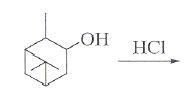
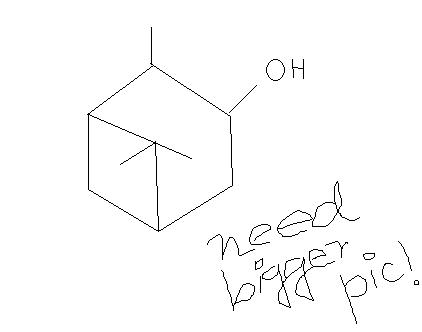
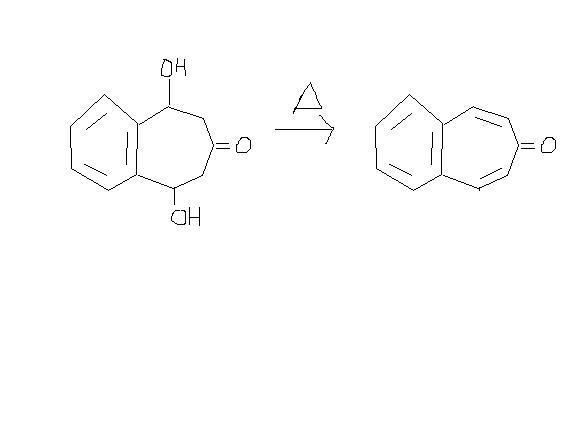
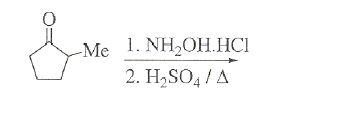3. won't the -Me group be oxidised by HNO3[7]
22 Answers
i was trying to find an excuse to avoid confusion...[4]
3.....why NO2 will go to only one of the rings...but not both????[4]
skygirl
dekha tumne ?
Sent at 20:22 on Thursday
Vishal
yaa 3rd wala dekha
Q 3
i think u r rite
skygirl
k kya hoga pata hai ?
hmm..
wat abt the point akki raised ?
i dun think steric thing will affect so much dat it will go and attack the less e-density wala ring ...
isnt it ?
waise i am not 100% sure
Sent at 20:26 on Thursday
Vishal
but o- has reso with C=O too this can pose a prob
kya hua
skygirl
koi prob nahi hoga
kaha kuchh hua:P
arey dekho
O- is in resonance
but LHS wale ring mein KOI PROBABILITY HA I HI NAHI
lone pair donation ka
Vishal
hmmm lag to raha hai
mere hisab se jyada se jyada methly gp replace ho sakta hai NO2 se
skygirl
nahi yaar
methyl grp is not a good leaving group
how can it be kicked off ?
Vishal
forced to leave due to hindrance
skygirl
baapre !
Vishal
but not enough hindrance here
skygirl
itna hindrance kaha hai
Vishal
so not possible
skygirl
this is TERRORISM!
:P
nai Aki it shud go to RHS ring although it has hinderance
sometimes even already occupid pos is replaced by NO2
but as O is also accociated with C=O reso so i think this may cause sm problems
lemme think more
@skygirl: wont the stearic hinderence at the ortho of the rhs effect the addition as there is bulky group present there and that too is undergoing single saturated bond rotation!!!!!!
cheers!!!!!!!!!
3.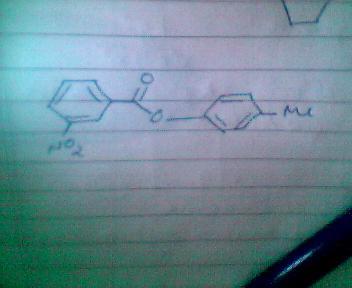
trying others! if this is not correct then tell!!!!
will see you soon after dinner!
hey abhirup, pls post a bigger pcture of the fourth one naa re... plz...
3) NO2+ will be generated which will go to that ring in which electron density is more.
clearly RHS ring has hogher e- density.
in this RHS wala ring ... prefrable sites are o-p positions.
but since the para position is occupied, NO2+ will go to the ortho postion.