 39
39Sorry, I felt sick, I had to get off.
Well, you guys have solved most of it. In Q1, I don't understand whether we need a trans or cis reducing agent, it doesn't specify the hydrogens' orientation in the end product. So I'll take Lindlar's catalyst..
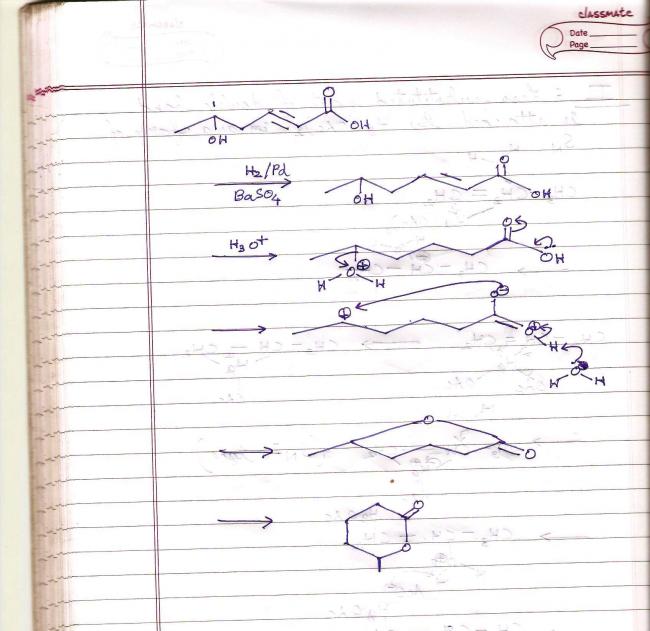
Missed out the double bond in the end waala, but you get the gist..
 1
1ans of 2nd: it shows +ve iodoform test.
After claisen, we will get: CH3 CO CH2 COOC2H5. With H+ and Na+ it will give: CH3-CO CH2COOH and CH3CH2ONa (purely exceptional case of alcohol).
Now, CH3CH2OH gives +ve iodoform and heat produced in rxn will decarboxylate beta keto ester and will give CH3COCH3 which also gives +ve iodo.
You both got it right and just while discussing this I cracked it together with you all...thnx.
Now will anyone spot-light q1?.. Pritish is right bt I cant c hw it helps in rxn
 3
3ankur .. im confused with 2 .........
i think th answer shud be it will show iodoform if it decarboxylates. ....... which usually happenes on heatin it .......
but if its just claisen ... then i guess the acidic hydrogen will be on the non terminal carbon ... so no iodoform ...
butw wats the answer ...
 1
1yeah.. u both are correct...
 106
106me too getting (3) first in presence of H+ the alkene will cyclise then acetic acid's OH will attack the positive charge with elimination of H+ to form (3)
 3
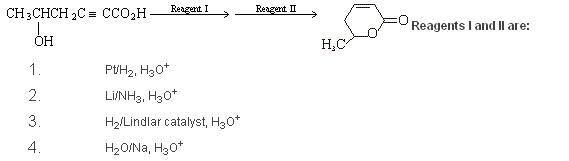
3Q1 is it 2 ......[nt sure ...][if possible organic mania or aieee or pritish or any 1 else pls list the properties of all the above reagents as to wat they reduce and wat they dont touch ....... or provide links ...]
basically the first reagent should be such that it reduces the triple bond but dosnt touch the carboxlic acid group .............
Q2 i think it should NOT GIVEEDITED SORRY ....CORRECTD BY IOTA .. iodoform as the most acidic hydrogens are in the terminal catbon[left side] ....'
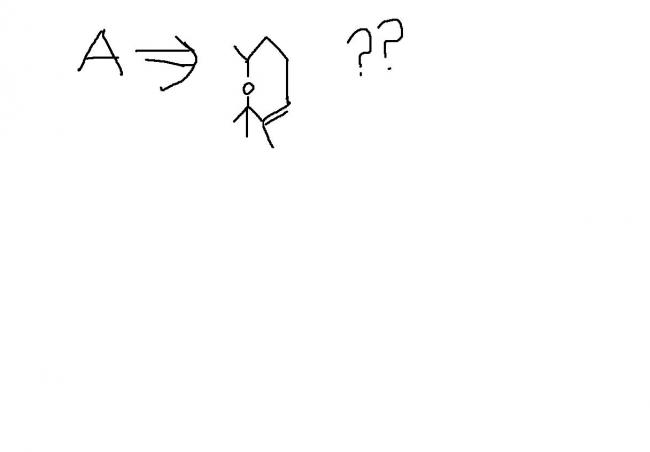
 1
1fantastic iitcomin.. I forgot all abt Claisen even when I was reading about Dieckmann dis morning...
for III one..dere are.. do u want?
 3
3any options provided for third 1 ankur???.......
 1
1@iitcomin ... ur Q3-A doesn't matches at all...show mecn
 1
1sorry.. Q2 : 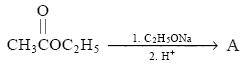 . Do A gives iodoform?
. Do A gives iodoform?
 13
131) Wht difference wud it make on using H2/Lindlar's as compared Li/NH3 ?
How is Syn/Anti addition affecting here ?
Ans : Either of 2 & 3
2) Will not give IodoForm... as the most Acidic Hydrogen is not the one of the -CH3 group....
3) Am also thinking...!
 39
39Finally on the comp....
Will solve these. Q2 mein the ester does not give iodoform test. Acetic acid derivatives do not give the test as in the mechanism acidic alpha hydrogens will not be left.
In Q1 they seem reducing agents to me....
Li/NH3 is a trans hydrogenating agent. It reduces alkynes to alkenes, and alkenes to alkanes. The other hydrogenating agents are syn.
 1
1I think Q1) is b
Q2) will not give iodoform test.
Q3) thinking...but i'm sure someone will give the answer before, as always.
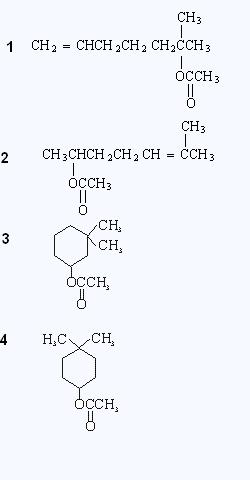
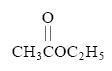 give Iodoform Test? [questio edited.. see below]
give Iodoform Test? [questio edited.. see below]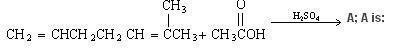

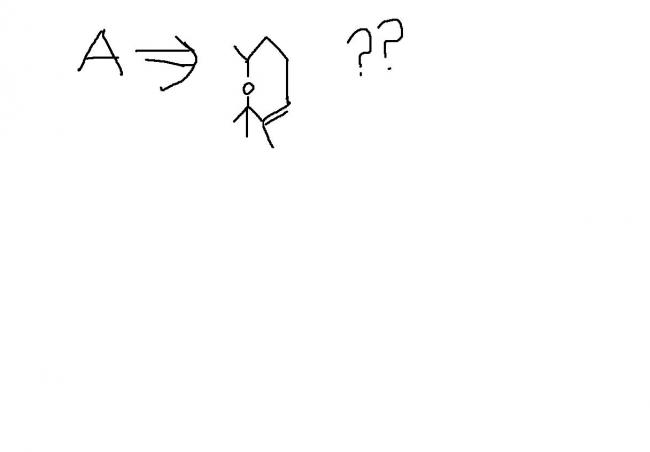
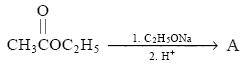 . Do A gives iodoform?
. Do A gives iodoform?