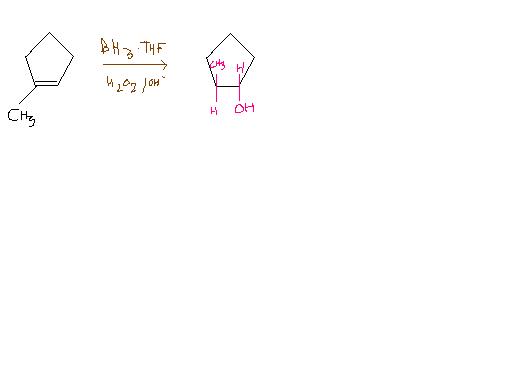23
23Q7- p- nitrophenol ??
in o-nitrophenol , H is busy in doing intra H bonding ,
in anion forms of their acids , delocalisation is more in para nitrophenol than in meta nitrophenol
, hence conjugate acid i.e para is more acidic than meta
and p-chloro phenol me to bas I effect hi hai
 11
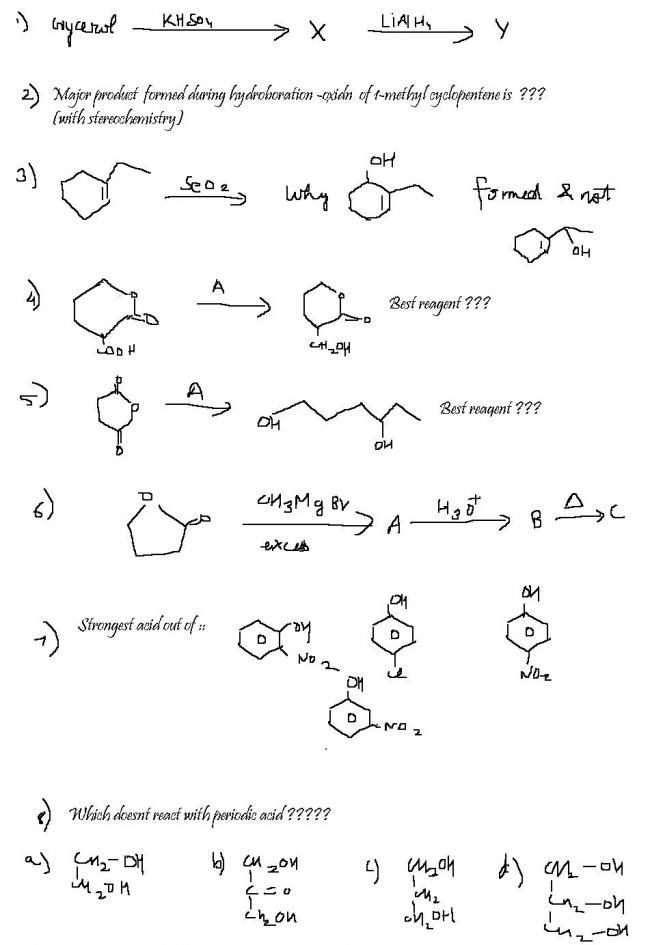
11Ans 1) X → CH2 = CH - CH = O (Acrolein)
Y → CH2 = CH - CH 2 OH
KHSO4 → Dehydration
Ans 2) 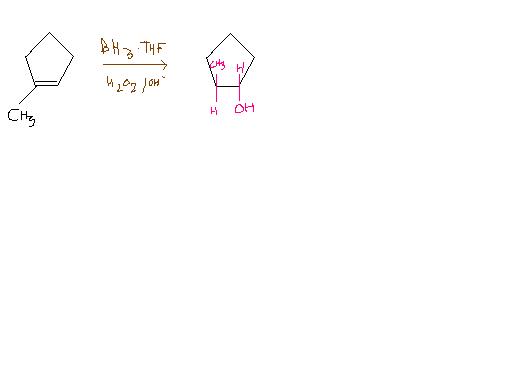
In hydroboration oxidation, the less sterically hindered carbon of the double bond gets preferrentially attatched to the hydroxyl group. Hydroboration oxidation is a syn addition.
So, the reqd ans is trans - 2- methyl - 1 - cyclopentanol.
Ans 3) This is allylic hydroxylation, the hydroxylation takes place at "Allylic" position, and not at benzylic position.
Ans 5) Li Al H4
This is bcoz NaBH4 cannot reduce carboxylic acid and ester but Li Al H4 can reduce all of them.
Ans 6) 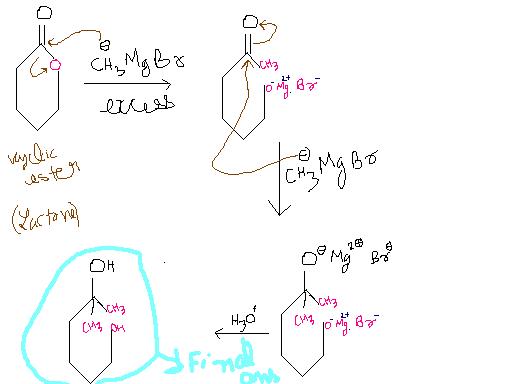
Ans 8) r the given options correct ??? I think ans shuld be :-
 106
106Q7. Strongest acid is p-nitrophenol.
First Cl is the weakest deactivator
then NO2 is electron withdrawing and it has -R and -I effect.
-R is most effective when NO2 is at o,p position.
but in ortho position H-bonding is possible which reduces acidity
So, p-nitrophenol is most acidic
 106
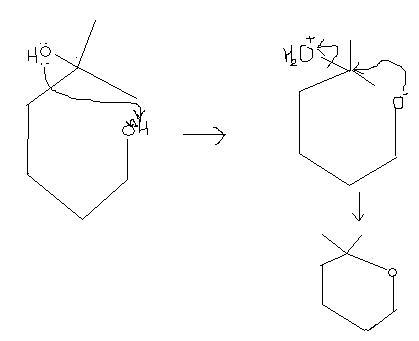
106Q6. reaction can proceed further as heat is being provided..
continuing from tush's product,
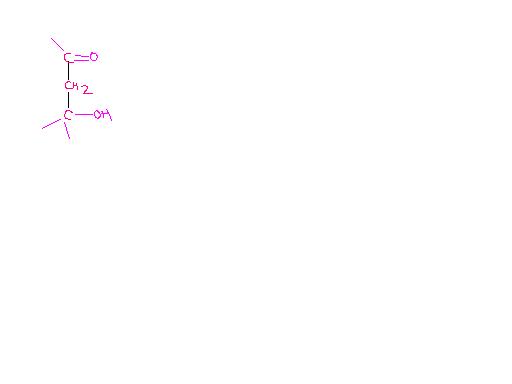
 11
11oh yeah u r correct Asish,
I forgot abt product C [2]
 24
24thx all........
but some dbts which remain ..
1)whats role of KHSO4 ?
3)@tush..couldnt understand the explaination
4) reason plzz
5)thx....dont know why ans was B2H6....it was worn obvously
8)ya options are rite
 11
11@eure
Ques 1, 3 done with explanation
No idea abt ques 8
 39
398)Per-iodic acid, or HIO4, oxidises only cis vicinal diols. So c) will not get oxidised as the diol is not vicinal, cis ki baat toh chorho hi. b) does react though. >C(-OH)- gets oxidised if >C=O is vicinal to it.
Note : Lead tetraacetate or Pb(OAc)4 can oxidise both cis and trans vicinal diols.