ya sngp
bt meko woh ni pata
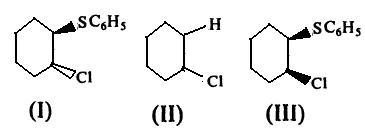
Which of the following is the correct order of rate of solvolysis of the above ?
(A) I < II << III (B) I >> II > III
(C) I ≈ III > II (D) I ≈ III <II
se after solvosis the charge on sulpher is - which is stablize by benzene ring by resonance
ohh cool big xplanation !
fm which boook u got dis image ?
morrison boyd kyaaaaa ?
ya....your ans was rite but i dont think steric effect plays any role here.It's basically due to "neighbouring grp.effect"
yeah the explanation to this cud be given by the neighbouring group effect...btw mates is all this stuff really there in our syllabus??
I guess all of you have different reasonings
BUT the correct ans is B i.e I >> II > III
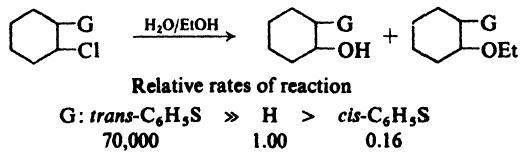
The explanation's coming ..........
dude !
wherez benzene?
it is cyclo hexane !!!!
u donnno diffn betn
benzene n cyclo hexane o wat ?
c6h6 benzene
c6h12 cyclo hexane
where can u see double bonds in CARBON?>? haaaaaaaa ?
either c or d
i go with
C
RKRISH ?????
SPILLDABEANSNOW !
pls temme wts solvolysis???????/
pls pls pls.......;-)
get d sol fr dis frm someone else.. but pls do explain me..
n dude !
ter is no benxene ??
kaha pe bbenzene dikh raha hai be ??
it is cyclo hexane !!!!!!!!!
ITS RIGHT
BETWEEN C N D
MANIPAL
BUT I THINK ITS C
Y U THINKK D?
rkrish pls tell da answer !!!!!!!
accordin 2 meh i feel answer is b cuz elimination takes place in trans way so 1 structure mein trans hai .so itna hindernce ni hoga weras last mein steric hindrnce hoga
corrct meh if m wrng
ab mere vichaar bhi sun lo ghante ki behass ke baad
the fight is b/w C and D
i think
the answer must be D
my reason C6H5 jo hai woh solvolysis mein hindrance daalega
benjene is non polar na and doesnt solvate easily
Solvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule.
For certain nucleophiles, there are specific terms for the type of solvolysis reaction.
For water, the term is hydrolysis; for alcohols, it is alcoholysis; for ammonia, it is ammonolysis.
i think its C ?
CHLORINE WILL not get easily brekoff
so as it will require solvents and other special reagents !
while 1 n 3rd r almost ~same
so........lone pair of e- on cl n as well due 2 lack of resonance it will adjust by elimination
so opti9on ONLY
C OR D
BUT FINAL I THINK SOO
C ?
is it right i just think so , pls scrap me if u know final !!111
and wat do u mean by " H n Cl jyaada water molecules ko attrct karenge " .
Solvolysis hai ..toh Cl has 2 b removed.
Speaking of lesser charge density , wat abt the electrons on S.. of PhS- ??