 106
106in gaseous phase.. only inductive effect is operative and more ERG increases basicity so the order is N-Me3 > NH-Me2 > NH2Me>NH3
In aq solution phase there is additional factor of H-bonding. now more H bond is possible if more N-H are there. so there is a steric factor involved.. Hence if we mix those two factors we get order of basicity as NHMe2 > NH-Me2 > N-Me3 > NH3
 1
1Due to + I effect of alkyl gps, the electron density on the N-atom of 1°, 2° and 3° amines is higher than that on the N-atom in NH3. Therefore, all amines are more basic than NH3.
(i) In gaseous phase, solvation effects are absent and hence the relative basicity of amines depends only on + I effect of the alkyl gps. Now since + I effect increases in going from 1° to 2° to 3° amine, so the basicity of amines decreases in the order :
3° amine > 2° amine > 1° amine
(CH3)3N > (CH3)2NH > CH3NH2 > NH3
(ii) In aq. soln, the basicity depends upon two factors :
(a) + I effect of CH3 gp and
(b) Solvation effect.
Stabilization of the conjugate acid (formed addition of a proton to amine) by H bonding explained above on the basis of + I effect, the order will be :
(CH3)3N > (CH3)2NH > CH3NH2
On the basis of Stabilisation of conjugate acids by H-bonding alone as explained below :
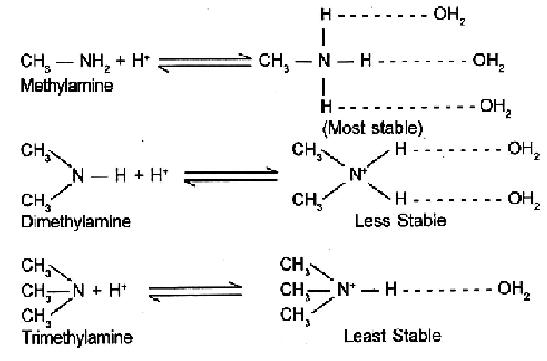
The combined effect of these two opposing factors is that (CH3)2 NH is the strongest base. In case of CH3NH2 and (CH3)3 NH, the stability due to H-bonding predeminates over stability due to + I effect of CH3 gp, thereby making CH3NH2 stronger than (CH3)3 NH. So the overall order in aq. soln will be :
(CH3)2 NH > CH3NH2 > (CH3)3N > NH3
 1
1@ Ashish
You are absolutely correct...no doubt
but I am giving detail explanation for others ..so that they can understand well.
 19
19an exactly same topic was exploited in FIITJEE open test last year, in a comprehension format !
 1
1I have not seen it !!!!!!
may be !!
