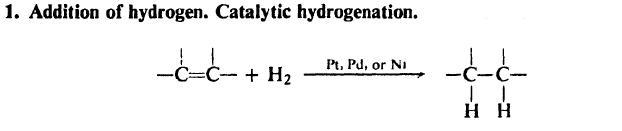yaar rkrish H2/Ni or Pd will reduce it yaar(it would be better if Ni or pd are finely Divided )........:P.........
What reagents (think of all possible reagents that can be used) which can perform this conversion successfully ?
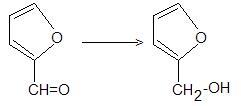
I know three;lets see how many you can think of !![6][6]
-
UP 0 DOWN 0 0 19

19 Answers
no i dun think here it will.......
cause if H+ ataches to O, any one bond will break.. resulting in + charge in one of the double bonded carbons..
which is very very unstable..
how about NaBH4 + H+ ... i am not sure whether it will reduce the O in the chain ...
krish u didnt get me i meant pd wont reduce CHO but NI wud...
@MATRIX...
for post #9...
I know that "...(Evrybody nows that ).:P..." BUT
HOW CAN YOU USE A GRIGNARD REAGENT WITH THE GIVEN SUBSTRATE,TO GET A 1° ALCOHOL ?????????????
for reaction with Grignards Reagent it will be a form a complex or in other words an "Adduct"........and on further reaction with water it will form the correspondin aldehyde and give back Grignards Reagent.........
and Grignards Reagent General Formula----->RMg(OH)X(Evrybody nows that ).:P...[3][3][3].....
hey rkrish this is a reduction process .........reduction of a alcoholic group to aldehyde group so for redution process to take place we need reducing agents.......
@MATRIX...
1)post #2 : can you elaborate a bit?
2)post #3 : H2/Ni or Pd will also reduce C=C
3)post #4 : can you give specific examples?
We can use any(not sure any may be some) mild Reducing Reagents for this Reduction Purpose........
Then the Second one is add H2 to alcohol in Presence of Finely divided Palladium or Nickel.....