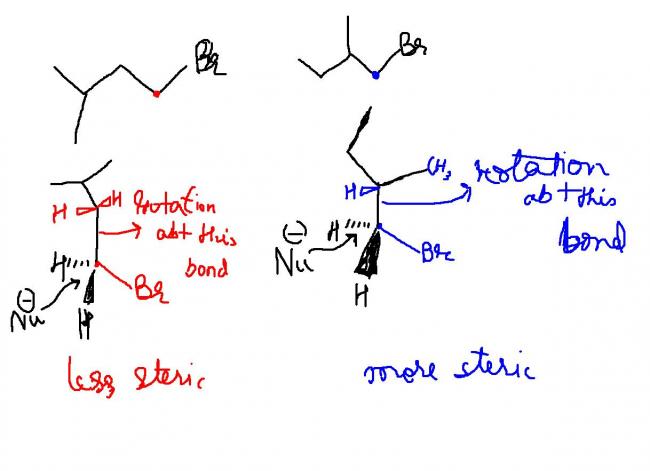
reason is as there is possiblity of compound 2 changing to 2° carbocation so SN1 will be more in compound 2

i m with first compound>!!!!!!!!!!! wat do u think????????
-
UP 0 DOWN 0 0 18

18 Answers
answer is first one according to me lesser steric factor here seems to be the key to finding the answer.
@kamalendu
bring me a clear macroscopic molecule of both to minutely analyze steric hinderance......kya yaar how can u say there is clearly more crowding in second case
no no.. there is clearly more crowding in second case ....asfor ur post #10 i dunno ..i only used those reasons in ethers.. not in halides...
bro....i don think its steric factor......each one is equally bulky(as each one is single branched[1])
neways.review my post #10.do u agree to it???????
sorry for the earlier casual error....... but still ans wud be the 1st one for Sn2 due to more in the second one which will hinder backside attack stearic factor!!
my reason favouring first one:
+I effect in carbocation of second compound is more than in 1st one.so,2nd compound will favour Sn1 and first one will favour Sn2.
hope u agree!!!!!!!!!!!
@kamalendu
so wat...................both r primary halides.....din u notice that!!!!!!!
ans ncert mein hi hain john.... primari halide is more likely to go for sn2
second one will result in 30 carbocation formation by hydride shift!!
it is first...
in second case there will be a hydride shift and tertiary carbocation formation...therefore sn1 is preferred
In first no such rearrangement, and primary halide..therefore sn2...
:)
@kamalendu(ques. rat ke rakhe ho kya......ans. not given[2])
give ur reason!!!!!
i don think steric hinderance would be too different in both!!!!!!!!