CH3 ke hypeconjugation ke wajah se the N atom will be more basic, enough to react in an acid base reaction with NaHCO3.
NaNO2/HCl will not work as there are no hydrogens present on N to facilitate diazotization. Will check about the beta-napthol waala and let you know.
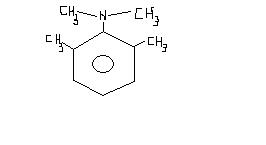
Can this give positive test with
1) NaHCO3 &
2) NaNO2 / H c l , β -napthol / OH-
ONE OR MORE THAN ONE OPTION MAY BE CORRECT
-
UP 0 DOWN 0 0 12

12 Answers
Pritish..the basicity of N will be reduced due to crowding by the two methyl groups and the phenyl group.
So the lone pair of N will not have enough place to leave the atom.
Therefore it may not react with NaHCO3.
Acid base reactions are very fast. Steric hindrance does not affect acid base reactions. That is why when a nucleophile is bulky, it will abstract a proton and lead to elimination in a substrate rather than substitution.
and the compound will give positive test with NaNO2 /HCl. I've shown the product..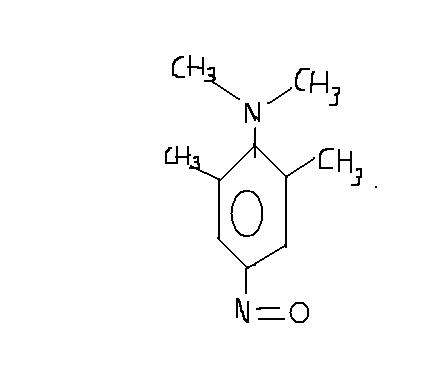
aieee....Activeness of lone pair is reduced due to stearic hindrance. That is why (CH3)3N is less basic than (CH3)2NH.
+I effect of alkyl groups raises the energy of the lone pair of electrons, thus elevating the basicity. Thus the basicity of an amine may be expected to increase with the number of alkyl groups on the amine. However, there is no strict trend in this regard, as basicity is also governed by other factors mentioned above. Consider the Kb values of the methyl amines given above. The increase in Kb from methylamine to dimethylamine may be attributed to +I effect; however, there is a decrease from dimethylamine to trimethyl amine due to the predominance of steric hindrance offered by the three methyl groups to the approaching Lewis acid.
Source - Wiki
Yes, prongs is right in that specific case.
And about NaNO2, I forgot about nitrosamine EAS..lol.
Thanks everyone the ans is
YES for NaHCO3
NO for NaNO2 / H c l , β -napthol / OH-
thnx for explaining pritish..
Uttara says that it doesn't give positive test with NaNO2/HCl. But I read that it would give a product as i've shown above.
So wat should I accept as the correct reaction? I'm getting confused.
Maybe because the electrophilic aromatic substitution with nitrosamine does not give indication of a product like a ppt or a colour?